Convert 22.7 grams of H2O to molecules
Answers
Answer: 1.2600414759026783 mol
Explanation:
Related Questions
Label with at least four types of energy (where does the energy flow and how)
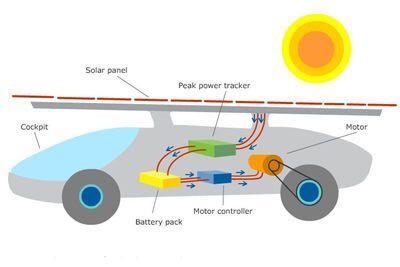
Answers
Answer:
The solar panel uses they sun as energy making the car work
Explanation:
Also it can store energy to
.What is the mass percentage of cobalt in cobalt(II) fluoride, CoF2?
Answers
Find the mass of cobalt is 12 and the mass of fluoride which is 19 and then add them which will be 50.
Since you're looking for Cobalt only, it will be 12 times 100% divided by 50 which will then give the percentage of 24%.
*why divide by 50, because it is the mass of the whole chemical.
The mass percentage of cobalt in Cobalt(II) fluoride is 60.8%
From the question,
We are to determine the mass percentage of Cobalt (Co) in Cobalt(II) fluoride (CoF₂)
The mass percentage of Cobalt in the Cobalt(II) fluoride can be determined using the formula
\(Mass\ percentage\ of\ Co= \frac{Atomic\ mass\ of\ Co}{Molar\ mass\ of\ CoF_{2} } \times 100\%\)
Atomic mass of Co = 58.93 g/mol
Molar mass of CoF₂ = 96.93 g/mol
∴ Mass percentage of Co = \(\frac{58.93}{96.93}\times 100\%\)
Mass percentage of Co = \(\frac{5893}{96.93}\%\)
Mass percentage of Co = 60.79645%
Mass percentage of Co ≅ 60.8%
Hence, the mass percentage of cobalt in Cobalt(II) fluoride is 60.8%
Learn more here: https://brainly.com/question/2789100
Hey y’all is these answers correct?

Answers
Answer:
ʏᴇs, ɪ ᴛʜɪɴᴋ
Explanation:
TᕼᗩᑎKՏ ᒪOᗪՏ
source: i’m black
Describe the difference between an atomic element and a molecular element. Check all that apply. Check all that apply. Molecular elements exist with a single atom as their basic unit. Atomic elements exist with a single atom as their basic unit. H2, F2, and P4 are some examples of atomic elements. H, F, and P are some examples of molecular elements. Atomic elements have more than one atom in their basic unit. H, F, and P are some examples of atomic elements. H2, F2, and P4 are some examples of molecular elements. Molecular elements have more than one atom in their basic unit.
Answers
Answer:
Atomic elements exist with a single atom as their basic unit.
H, F, and P are some examples of atomic elements.
H2, F2, and P4 are some examples of molecular elements.
Molecular elements have more than one atom in their basic unit.
Explanation:
Atomic element exists as the smallest particle of an element that can take part in a chemical reaction. They exist with a single atom as their basic unit. The atomic number of an element is the number of protons present in the atomic nucleus of the element. Atomic elements are usually monoatomic such as H, F and P.
Molecular elements are the smallest particle that are capable of independent existence and still retains the chemical properties of those substances. They are capable of independent existence because they are diatomic. The combination of atoms of the same type produces a molecule of an element such as H₂, F₂, and P₂.
using cahn-ingold-prelog rules, identify the substituents that would have the highest priority. a) i b) ii c) iii d) iv
Answers
Using Cahn-Ingold-prelog rules, the substituents that would have the highest priority is a) i
According to the Cahn Ingold Prelog system, priorities are assigned as 1,2,3, or 4 to the atoms directly bonded to the stereo genic center in decreasing order of atomic number. The atom of highest atomic number has the highest priority 1.
A substituent with higher atomic number takes over a substituent with a lower atomic number. Hydrogen is the lowest priority substituent, as it has the lowest atomic number. But for isotopes, the atom with the higher atomic mass gets higher priority.
For assigning the priority, multiple bonds are seen as if each bond of the multiple bond is bonded to an unique atom.
To know more about Cahn-ingold-prelog rules, refer
https://brainly.com/question/16833491
#SPJ4
the hypothesis can be accepted because the electromagnet with the longer cooper wire had great strength
Answers
I uh- I'm a bit confused- W h a t
Hello people ~
Which of the following fibres is used for making parachutes?
A. Plastic
B. Terelyne
C. Nylon
D. Taylor swift
Answers
Answer:
C. Nylon
Explanation:
Nylon is a synthetic fiber, strong, elastic, and holds excellent abrasion resistance. Therefore it is used for making parachutes and ropes for rock climbing.
Answer:
Nylon
Explanation:
Nylon is a synthetic fibre not natural so long lastingIt is highly elastic and strong material.It is water proof also so used for making parachutesHow many neutrons does Ruthenium have?
Answers
Answer: 57 neutrons
Explanation: Ruthenium has an atomic number of 44, meaning it has 44 electrons, 44 protons, and 57 neutrons.
Answer:
57 neutrons
Explanation:
Ruthenium has atomic number of 44, that is, it contains 44 electrons distributed in atomic orbitals and its nucleus has 44 protons and 57 neutrons (Figure 1). Electron distribution in atomic or molecular orbitals is called electron configuration which for Ru and the other group 8 chemical elements is shown in Table 1.
After siting down the C(2)-C(3) bond in the Newman projection of
butane, which is the gauche conformation? H₂C H H H H H₂C H CH₂ H H
CH₂ (d) H H₂C H H Ka H CH3 H (b) H H- CH₂ H H₂C H H H
Answers
The gauche conformation after rotating the C(2)-C(3) bond in the Newman projection of butane is represented by option (b) H H- CH₂ H H₂C H H H.
In the Newman projection, the carbon atoms are represented by the intersecting lines, and the substituents are represented by the groups attached to each carbon. By rotating the C(2)-C(3) bond, we can determine the gauche conformation, which refers to the steric interaction between two larger groups positioned at a dihedral angle of approximately 60 degrees.
In the given options, option (b) shows the gauche conformation because the two larger groups, represented by H and CH₂, are positioned on adjacent carbons with a dihedral angle close to 60 degrees. This arrangement results in steric strain due to the repulsive interactions between the electron clouds of these groups.The other options do not exhibit the gauche conformation. In option (d), the H and CH₃ groups are not adjacent to each other, so they do not contribute to steric strain. Option (a) shows an anti conformation, where the two larger groups are positioned on opposite sides of the carbon-carbon bond, resulting in minimal steric strain. Option (c) represents an eclipsed conformation, where the two larger groups are directly aligned, leading to maximum steric strain.In summary, option (b) represents the gauche conformation after rotating the C(2)-C(3) bond in the Newman projection of butane.
Learn more about: Newman projection
brainly.com/question/24608677
#SPJ11
draw detailed mechanisms for the formation of both products, including any resonance contributors. (6 pts) b) briefly explain why pinacolone formation is favored. (4 pts)
Answers
To address the student question, I will provide a step-by-step explanation for drawing the detailed mechanisms for the formation of both products, including any resonance contributors, and then explain why pinacolone formation is favored.
What is the mechanism of product formation?
Step 1: Start with the pinacol molecule, which has two hydroxyl groups on adjacent carbons.
Step 2: Add an acid catalyst (e.g., H+) to the reaction.
The acid will protonate one of the hydroxyl groups, turning it into a better-leaving group (H2O).
Step 3: The protonated hydroxyl group leaves, generating a carbocation at the carbon it was attached to.
This carbocation can have resonance contributors if there are double bonds or lone pairs on adjacent atoms.
Step 4: The remaining hydroxyl group donates its lone pair of electrons to form a bond with the carbocation, resulting in a cyclic transition state.
Step 5: A molecule of water acts as a base, abstracting a proton from the newly formed bond, leading to the formation of pinacolone and water.
Step 6: The alternative product, pinacol, can form by following similar steps, but with a different leaving group and carbocation intermediate.
b) Pinacolone formation is favored because of the stability of the carbocation intermediate.
The carbocation generated during the pinacol rearrangement is more stable due to resonance contributors and the inductive effect of the alkyl groups, which help distribute the positive charge.
Additionally, the formation of pinacolone is thermodynamically favored, as it is a more stable molecule than the alternative product.
To know more about the Pinacol molecule, visit:
https://brainly.com/question/29507965
#SPJ11
A treasure chest is full of coins. The chest has a volume of 2.25L. The coins have a combined mass of 48kg. Calculate the density of the coins to determine which metal they’re made of. Which metal are they?
a. Silver: 10.5 g/mL
b. Lead: 11.3 g/mL
c. Bronze: 8.7 g/mL
d. Gold: 19.3 g/mL
Answers
The density of the coin, given the data from the question is 21.33 g/mL.
What is density?The density of a substance is simply defined as the mass of the subtance per unit volume of the substance. Mathematically, it can be expressed as
Density = mass / volume
How to determine the density of the coinFrom the question given above, the following data were obtained:
Volume of coin = 2.25 L = 2.25 × 1000 = 2250 mLMass of coin = 48 Kg = 48 × 1000 = 48000 gDensity of coin =?Density = mass / volume
Density = 48000 / 2250
Density = 21.33 g/mL
How to determine the metalThe density of the coin obtained, given the data from the question is 21.33 g/mL
Considering the options given, the density of the metal is not specified.
Learn more about density:
https://brainly.com/question/952755
#SPJ1
A 748 ml sample of H2 gas is collected over water at 26 degrees C and 742 mm Hg pressure. Calculate the grams of gas collected. The vapor pressure of water is 25 mm Hg at 26 degrees C
Answers
The mass of H2 gas collected is 0.0561 g. To calculate the mass of H2 gas collected, we need to first correct the measured pressure for the presence of water vapor.
The pressure of the water vapor is 25 mm Hg, so the partial pressure of H2 gas is:
P_H2 = P_total - P_water
P_H2 = 742 mm Hg - 25 mm Hg
P_H2 = 717 mm Hg
Next, we need to use the ideal gas law to calculate the number of moles of H2 gas:
PV = nRT
where P is the partial pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the gas constant, and T is the temperature in Kelvin.
Converting the given temperature to Kelvin:
T = 26 + 273 = 299 K
Substituting the given values:
n = (P_H2 * V) / (R * T)
where R = 0.0821 Latm/(molK) is the gas constant.
n = (717 mm Hg * 0.748 L) / (0.0821 Latm/(molK) * 299 K)
n = 0.0279 mol
Finally, we can use the molar mass of H2 gas (2.016 g/mol) to calculate the mass of the gas collected:
mass = n * molar mass
mass = 0.0279 mol * 2.016 g/mol
mass = 0.0561 g
Hydrogen gas, or H2, is a diatomic molecule made up of two hydrogen atoms. It is the lightest and most abundant element in the universe. H2 is colorless, odorless, and highly flammable in the presence of air, oxygen, or heat. It is commonly used in industrial processes, such as the production of ammonia and methanol, as well as in fuel cells for powering vehicles and generating electricity.
Learn more about H2 gas here:
https://brainly.com/question/29006846
#SPJ11
Au HSO3 nomenclatura
Answers
helppppppppppp pls lol

Answers
Answer:
Have a good day Ahead
:)))))))))((

Answer:
Electrical to mechanical
Explanation:
Unless of course the motor is gasoline or deisel.
global warming is caused by an increase in the level of carbon dixode gas in the atmosphere. How has this affected the world's oceans?
Answers
Which toxic substance is often used to extract gold and results in harmful environmental effects?
a. acid mine drainage
b. carbon dioxide
c. sulfur dioxide
d. cyanide
e. fluoride
Answers
how many liters of o2 at 800 torr and 25c would be produced by decomposition of a 25g sample of 65% pure kmno4
Answers
The decomposition of 25g of 65% pure KMnO4 at 25°C and 800 torrs of oxygen will produce 0.67 liters of oxygen gas.
The decomposition of 25g of 65% pure KMnO4 at 25°C and 800 torrs of oxygen can produce a significant amount of oxygen gas. To calculate the exact amount of oxygen produced, we need to use the ideal gas law. The ideal gas law states that the volume of a gas is proportional to its temperature and pressure, and inversely proportional to its molar mass.
Using this equation, we can calculate the number of moles of oxygen produced by the decomposition of KMnO4. First, we need to determine the molar mass of KMnO4.
The molar mass of KMnO4 is 158.0 g/mol. That means that 25g of KMnO4 has a molar mass of 0.158 mol. To determine the amount of oxygen produced, we need to multiply the molar mass of KMnO4 by its oxygen content, which is 65%. So, 0.158 mol x 0.65 = 0.1017 mol of oxygen produced.
Now that we have the number of moles of oxygen produced, we can use the ideal gas law to determine the volume of oxygen produced. Therefore, the volume of oxygen produced (in liters) is equal to 0.1017 mol x 800 torrs x 25°C/158.0 g/mol = 0.67 liters.
Therefore, the decomposition of 25g of 65% pure KMnO4 at 25°C and 800 torrs of oxygen will produce 0.67 liters of oxygen gas.
Learn more about KMnO4:
brainly.com/question/29555671
#SPJ4
PLEASE HELP IM BEGGING YOU!!!
What is the mole ratio of carbon dioxide to water? 2C2H8 + 7O2 ->4H2O + 6CO2 7 mol : 4 mol
6 mol : 4 mol
6 mol : 2 mol
7 mol : 6 mol
Answers
This means that for every 6 moles of CO2, there are 4 moles of H2O involved in the reaction.
The mole ratio of carbon dioxide (CO2) to water (H2O) can be determined by examining the balanced chemical equation:
2C2H8 + 7O2 -> 4H2O + 6CO2
From the equation, we can see that for every 6 moles of CO2 produced, 4 moles of H2O are also produced. Therefore, the correct mole ratio of carbon dioxide to water is:
6 mol : 4 mol
This means that for every 6 moles of CO2, there are 4 moles of H2O involved in the reaction.
To know more about balanced chemical equation click this link -
brainly.com/question/14072552
#SPJ11
The weight of a table as measured by a student is 20 kg.The
actual weight of the table is 23 kg. What is the percent error
in the student's measurement?
a 43 %
b 15 %
c 13%
d 3%
Answers
now the answer asks for percentage ERROR
100-87 =13
so answer is C
Which chemical equation correctly represents the reaction that takes place when nitrogen gas and hydrogen gas are formed as ammonia decomposes? (4 points) a N(g) + H3(g) → NH3(g) b 2N(g) + 3H2(g) → 2NH3(g) c 2NH3(g) → N2(g) + 2H3(g) d 2NH3(g) → N2(g) + 3H2(g)
Answers
Answer:
d. 2NH3(g)-N2(g)+3H2(g)Commercial grade hcl solutions are typically 39. 0% (by mass) hcl in water. Determine the molarity of the hcl if the solution has a density of 1. 20 g ml-1.
Answers
The molarity of a commercial grade of HCl solution with a density of 1.20 g/ml is 12.8 M.
What is molarity?The term "molar concentration" (also known as "molarity," "amount concentration," or "substance concentration") refers to the amount of a substance per unit volume of solution and is used to describe the concentration of a chemical species, specifically a solute, in a solution.
The most common measure of molarity in chemistry is the number of moles per litre, denoted by the unit symbol mol/L or mol/dm3 in SI units. A solution with a concentration of 1 mol/L is referred to as 1 molar, or 1 M. The most popular unit of measurement for molal concentration or molarity is moles of solute per litre of solution.
Learn more about molarity
https://brainly.com/question/14469428
#SPJ4
manganese-56 is a beta emitter with a half-life of 2.6 hours. what is the mass of manganese-56 in a 1.0mg sample remaining at the end of 10.4 hours?
Answers
The mass of manganese-56 in a 1.0mg sample remaining at the end of 10.4 hours is 0.0025mg
What is Half Life?
Half-life, in radioactivity, is the amount of time needed for half of a radioactive sample's atomic nuclei to decay (change spontaneously into other nuclear species by emitting particles and energy), or, alternatively, the amount of time needed for a radioactive material's rate of disintegrations per second to decrease by half.
Cobalt-60, a radioactive isotope used in radiotherapy, has a half-life of 5.26 years, for instance. As a result, after that time, a sample that contained 8 g of cobalt-60 at first would only have 4 g of cobalt-60 and would produce half as much radiation. Only 2 g of cobalt-60 would remain in the sample after an additional delay of 5.26 years. The initial sample's mass and volume don't change noticeably,
10.4/2.6= 4
The mass of manganese-56 in a 1.0mg sample remaining at the end of 10.4 hours is 0.0025mg
Learn more about half life from given link
https://brainly.com/question/25750315
#SPJ4
The list identifies various properties of four elements: element 1 is a gas at room temperature. Element 2 is a solid which conducts electricity. Element 3 is a gas with an effective nuclear charge of +7. Element 4 is malleable and can take the form of a shiny solid sheet. Based on this list, which elements are metals?.
Answers
An element is a basic object that is difficult to break down into smaller pieces/ malleable. As a result, choice D is right.
Describe element.An element is a species of atoms with a specified number of protons in their nuclei, including the pure substance comprised completely of that species. Contrary to chemical compounds, chemical elements cannot be broken down into smaller molecules through any chemical process.
A substance that cannot be broken down by non-nuclear processes in physics and chemistry is called an element. In computers and mathematics, an element is a discrete part of a larger system or collection.
The three major divisions of the Periodic Table are metals, nonmetals, and metalloids. The components of each category have similar physical and chemical properties. Among the physical characteristics used to separate the three groups are, A substance's capacity to reflect light is referred to as luster.
To learn more about elements visit:
brainly.com/question/13025901
#SPJ1
68.19g of magnesium phosphite from mass to grams
Answers
Magnesium phosphite is a white or off-white crystalline compound with a chemical formula of Mg3(PO3)2. It is a strong reducing agent and reacts violently with water, producing toxic fumes of phosphorus oxides. It is commonly used in the manufacture of fertilizers, insecticides, and herbicides.
In this problem, we have 68.19 g of magnesium phosphite, and we need to convert it to grams. The first step is to determine the molar mass of magnesium phosphite. Magnesium has a molar mass of 24.31 g/mol, phosphorus has a molar mass of 30.97 g/mol, and oxygen has a molar mass of 16.00 g/mol. Therefore, the molar mass of magnesium phosphite is:Mg3(PO3)2 = (3 x 24.31 g/mol) + (2 x 31.00 g/mol) + (6 x 16.00 g/mol) = 262.00 g/molNext, we can use the molar mass to convert the given mass of magnesium phosphite to moles:68.19 g Mg3(PO3)2 x (1 mol / 262.00 g) = 0.2605 mol Mg3(PO3)2Finally, we can convert moles to grams by multiplying by the molar mass:0.2605 mol Mg3(PO3)2 x 262.00 g/mol = 68.19 gTherefore, 68.19 g of magnesium phosphite is equal to 0.2605 moles of magnesium phosphite, and its mass is equal to 68.19 g.For such more question on moles
https://brainly.com/question/29367909
#SPJ8
what factors cause changes between the liquid and gas state?check all that apply.what factors cause changes between the liquid and gas state?check all that apply.a liquid can be converted to a gas by cooling.a gas can be converted into a liquid by heating.a gas can be converted into a liquid by cooling.a liquid can be converted to a gas by heating.a gas can be converted into a liquid by decreasing the pressure of a gas sample.a gas can be converted into a liquid by increasing the pressure of a gas sample.
Answers
A gas can be converted into a liquid by increasing the pressure of a gas sample cause changes between the liquid and gas state.
Option C is correct.
When the temperature and pressure of a system change, the system's state changes. Matter exists in three main states:
1) Strong
2) Fluid
3) Gas
At the point when the fluid is warmed the particles in the fluid addition dynamic energy or more a specific temperature, the particles escape from the fluid stage into the gas stage. As a result, heating is able to transform the liquid into the gas phase.
Gas stage :In the gas stage, the intermolecular power of attractions between the particles is exceptionally frail contrasted with that in the fluid stage. In the gas phase, the molecules are very far apart from one another. The intermolecular force between the molecules increases even more when the gas sample's pressure is raised. As a result, an increase in the gas sample's pressure can turn a gas into a liquid.
However, compared to the molecules in the gas phase, the molecules in the liquid phase have less energy and are closer to one another.
Incomplete question :
What factors cause changes between the liquid and gas state? Check all that apply.
A. A gas can be converted into a liquid by decreasing the pressure of a gas sample.
B. A liquid can be converted to a gas by heating.
C. A gas can be converted into a liquid by increasing the pressure of a gas sample.
D. A liquid can be converted to a gas by cooling.
E. A gas can be converted into a liquid by cooling.
F. A gas can be converted into a liquid by heating.
Learn more about Gas stage :
brainly.com/question/29767119
#SPJ4
what causes thermal energy to be released
Answers
Thermal energy (also called heat energy) is produced when a rise in temperature causes atoms and molecules to move faster and collide with each other. The energy that comes from the temperature of the heated substance is called thermal energy.
Hope this helps! :)
calculate the [h3o+] and ph of each polyprotic acid solution.
Answers
The H₃O⁺ is 0.380 M and the pH of this solution is 0.420.
To calculate the [H₃O⁺] (hydronium ion concentration) of a polyprotic acid solution, we need to consider the ionization steps of the acid.
H₃PO₄ is a polyprotic acid that ionizes in multiple steps:
H₃PO₄ ⇌ H⁺ + H₂PO₄⁻
H₂PO₄⁻ ⇌ H⁺ + HPO₄²⁻
HPO₄²- ⇌ H⁺ + PO₄³⁻
Since we are given the concentration of H₃PO₄, we can assume that it is fully ionized in the first step.
Therefore, the concentration of H⁺ from H₃PO₄ is equal to the concentration of H₃PO₄ itself.
[H₃O⁺] = [H⁺] = 0.380 M
So, the [H₃O⁺] is 0.380 M.
To calculate the pH of the solution, we can use the formula:
pH = -log[H₃O⁺]
pH = -log(0.380)
= -(-0.420)
= 0.420
To learn more on Polyprotic acid solution click:
https://brainly.com/question/31116483
#SPJ4
Calculate the [H3O+] of the following polyprotic acid solution: 0.380 M H3PO4. Express your answer using two significant figures. [H3O+] =
Calculate the pH of this solution. Express your answer using one decimal place. pH =
If 33.0 grams of hydrogen gas react with 300. grams of oxygen gas, what is the percent yield if an experiment produces 250. grams of water? Use the balanced equation from question 3.
Answers
Answer:
can you show us question 3 pls
Explanation:
How many cm3 in a L?
Answers
25.As a solution becomes more acidic, the pH of the solution...Select one:a. increases.b. decreases.c. remains unchanged.d. quickly increases and then gradually decreases.
Answers
Answer:
\(B\text{ : decreases}\)Explanation:
Here, we want to know what happens to a solution that becomes more acidic
A lesser ph (1-7) indicates acidity with the acidity being higher as the number becomes smaller
What this means is that a solution with a pH of 3 is more acidic than a solution with a pH of 5
Thus, when the acidity increases, it is expected that the pH of the solution decreases (it becomes smaller in number)