Answers
question two is "X" (the symbol)
Related Questions
two molecules with the same structural formula must have:
Answers
Which statement is true about the theory of plate tectonics and the theory of continental drift?
Answers
Answer:
The theory of plate tectonics explains how continental movements could occur
Explanation:
Answer:
The theory of plate tectonics does not explain how continental movements could occur.
Explanation:
Ruth rode home at a constant speed for the first 10 minutes of the trip. What was her constant speed?
______ m/min
What was Ruth's average speed for the entire trip?
______ m/min
Ruth stopped to talk with another friend during the trip.
How far was she from home when she stopped?
______ m
How long was she stopped?
_____ min
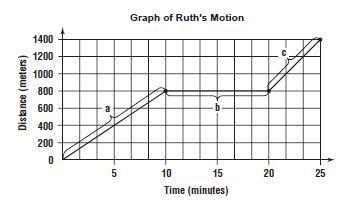
Answers
From the graph, the distance for the first 10 minutes is 600 meters.
10 minutes = 10 x 60 = 600 seconds
Ruth's speed for the first 10 minutes = distance/time
= 600/600 = 1 m/s
Average speed = total distance traveled/total time taken
Total distance = 1400 meters
Total time = 25 minutes = 25 x 60 = 1500 seconds
Average speed = 1400/1500 = 0.93 m/s
Ruth had traveled 800 meters before stopping to talk to her friend:
1400 - 800 = 600 meters.
Thus, she was 600 meters away from home when she stopped.
She stopped between 10 and 20 minutes:
20 - 10 = 10 minutes
Thus, she stopped to talk to her friend for 10 minutes.
More on velocity-time graphs can be found here: https://brainly.com/question/11290682
#SPJ1
is part of the digestive and the respiratory system because food and air can travel 3. The through it.
OLungs
O Heart
O Pharynx
O Diaphragm
Help me!!!
Answers
Answer:
pharynx
Explanation:
krypton has a completely blank p sublevel, giving it chemical stability. bromine needs one electron to achieve a completely filled p sublevel and thus has a highly blank electron affinity. therefore, it easily takes on an electron and is reduced to the bromide ion, giving it the added stability of the filled p sublevel.
Answers
Bromine is more reactive than krypton because of its electronic configuration. Bromine is a member of the halogen family, meaning it has seven valence electrons in its outermost shell. This makes bromine more reactive than krypton, which has eight valence electrons in its outermost shell.
Bromine has a higher reactivity because it is more willing to accept electrons, which allows it to form bonds with other elements more easily.
This is due to the fact that bromine has the ability to form a stable octet of electrons in its outermost shell by gaining one more electron as its valence shell has 4s²4p⁵.
On the other hand, krypton has a full outer shell of eight electrons 4s²4p⁶, and it is not as willing to accept additional electrons, making it less reactive. Therefore, bromine is more reactive than krypton in the periodic table.
To know more about halogens, click below:
https://brainly.com/question/1450671
#SPJ4
a molecule having four total electron groups around the central atom has three bonding electron groups and one nonbonding electron group. what is its molecular structure
Answers
A molecule having four total electron groups around the central atom has three bonding electron groups and one nonbonding electron group. Its molecular structure is trigonal pyramidal.
What do you understand by the term molecules?One or more atoms are joined by covalent (chemical) bonds to form molecules. Atoms can be represented as circles with a nucleus at their center (consisting of protons and neutrons) and one or more concentric circles around them, which represent the "shells" or "levels" in which the electrons surrounding the atom's nucleus are located, along with markings indicating the electron at each level. The lowest unit into which a substance can be divided while still being the same substance is a molecule. It is composed of two or more atoms that are chemically bonded to one another.
Learn more about an atom's nucleus here:-
https://brainly.com/question/26044300
#SPJ4
explain how can the periodic table be used to predict how many electrons a certain metal atom loses to form an ion in an ionic compound? provide two examples.
Answers
Predict how many electrons a certain metal atom loses to form an ion in an ionic compound we use the column that they are in to know how many valence electrons they have.
What kinds of metal are there?Ferrous metals, which include iron, and non-ferrous metals, which do not, are the two basic categories into which metals can be separated. Pure iron is too soft and ductile to be much use as just an engineering material.
Which are the 20 metal elements?Lithium, Beryllium, Sodium, Mg, Aluminum, Potassium, & Calcium are the metals within the first twenty elements. The first 20 elements' non-metals are currently Hydrogen, Helium, Carbo, Nitrogen, Oxygen, Fluorine, and Carbon.
To know more about metal visit:
https://brainly.com/question/12708186
#SPJ4
Calculate the energy of a photon emitted when an electron in a hydrogen atom undergoes a transition from =3 to =1.
Answers
Answer:
\(1.936\times 10^{-18}\ \text{J}\)
Explanation:
\(R_h\) = Rydberg constant = \(2.178\times 10^{-18}\ \text{J}\)
\(n_i\) = Initial shell = 3
\(n_f\) = Final shell = 1
We have the relation
\(\Delta E=R_h(\dfrac{1}{n_f^2}-\dfrac{1}{n_i^2})\\\Rightarrow \Delta E=2.178\times 10^{-18}(\dfrac{1}{1^2}-\dfrac{1}{3^2})\\\Rightarrow \Delta E=1.936\times 10^{-18}\ \text{J}\)
The energy of the photon emitted here is \(1.936\times 10^{-18}\ \text{J}\).
how much energy is needed to convert 120g of ice at -35°C to steam at 150°C?
Answers
if you gently shake a separatory funnel containing a carboxylic acid with ethyl acetate and aq nahco3, the organic acid will be found where? (multiple answers possible)
Answers
The organic compound, carboxylic acid will be found in ethyl acetate.
What are organic compounds?Organic compounds are those compounds that are obtained naturally from living matter.
Organic compounds are mostly composed of carbon, hydrogen, and oxygen.
Organic compounds are generally covalent compounds. Hence, they are usually non-polar compounds.
Based on the principle of like dissolves like, organic compounds are usually soluble in non-polar organic solvents and insoluble in polar inorganic solvents like water.
Carboxylic acid is an organic compound.
Ethyl acetate is an organic compound.
Aqueous NaHCO₃ is an inorganic compound.
Hence, carboxylic acid will dissolve in Ethyl acetate.
Learn more about organic compounds at: https://brainly.com/question/6279332
#SPJ1
How many ions are in 0.5 moles of NaCl?
Answers
Explanation:
Step 1: Write out the chemical formula of the compound.
Magnesium Chloride = MgCl^2
Step 2: Convert moles into number of compounds using Avogadro's number
0.5 moles MgCl^2 ⋅(6.022⋅10^23 - 1 mole MgCl^2)= 3.011⋅ 10^23
Step 3: Determine how many Chloride ions there will be in 1 compound
There will be 2 Cl− ions in each compound (Cl^2 part)
Step 4: Multiply the number from Step 2 by the number in Step 3
3.011 ⋅ 10^23 ⋅ 2= 6.022 ⋅ 10^23
We do this because the ratio is 2 Cl− ions for every 1 compound.
In the case of finding how many ions in general (both sodium and chloride), we would multiply by 3 because there are 3 ions per 1 compound.
I hope this helped!
1 mole of NaCl = 2 moles of ions, so 0.5 moles of NaCl contain 1 mole of ions.
Sodium chloride (NaCl) dissociates into sodium ions (Na⁺) and chloride ions (Cl⁻) in a 1:1 ratio. This means that for every mole of NaCl, you get one mole of sodium ions and one mole of chloride ions.
Therefore, 0.5 moles of NaCl will yield 0.5 moles of sodium ions and 0.5 moles of chloride ions. Since you asked about the total number of ions, you need to add the number of sodium ions and chloride ions together. As a result, 0.5 moles of NaCl will contain a total of 1 mole of ions.
The key factor here is the balanced stoichiometry of the dissociation reaction, which gives a 1:1 ratio of ions for NaCl.
To learn more about Sodium chloride here
https://brainly.com/question/9811771
#SPJ3
1.)Describe Lisa’s accuracy and precision and why
2.) Describe Lamont’s accuracy and precision and why
3.) Describe Liam’s accuracy and precision and why

Answers
Accuracy refers to how close the measurements that are made are to the real value.
What is accuracy and precision?The term accuracy refers to how close the measurements that are made are to the real value. The precision refers to how close the measurements are to each other in value. But precision and accuracy play a role in deciding whether or not a measurement is accurate.
Looking at the table;
Lisa's measurement is neither accurate nor preciseLamont's measurement is accurate and preciseLiam's measurement is precise but not accurateLearn more about accuracy and precision:https://brainly.com/question/15276983
#SPJ1
A _____________ __________________ occurs at the coast of oceans and other large water bodies during sunny days
Answers
A sea breeze occurs at the coast of oceans and other large water bodies during sunny days.
Where does sea breeze occur?The term sea breeze defined as it describes a wind that blows from the ocean inland towards land. In daytime, the land surface heats up faster than the water surface.
The sea breeze occurs mostly in the spring and summer months because of the higher temperature differences between the ocean and nearby land, mostly in the afternoon when the land is at more heating from the sun.
Thus, A sea breeze occurs at the coast of oceans and other large water bodies during sunny days.
To learn more about sea breeze, follow the link;
https://brainly.com/question/13015619
#SPJ1
why do we not do a melting point directly on camphor to assess its purity?
Answers
Camphor get sublimated when it is getting in contact into heat that is why we do not do a melting point on it.
Camphor isn't a suitable emulsion for determining its chastity by melting point because it has a fairly broad melting range and a high degree of sublimation, which can make it delicate to gain accurate and reproducible melting point data. Camphor has a melting point range of roughly 175- 180 °C, which is fairly broad compared to numerous other organic composites. This broad melting range can make it delicate to determine the precise melting point of camphor, which can affect the delicacy of assessing its chastity.
In addition, camphor has a high degree of sublimation, meaning it can decimate directly from its solid form to its gas phase without going through a liquid phase. This can beget crimes in determining the melting point because the sublimation of camphor can affect the appearance of the sample, similar as the conformation of recesses or craters on the face, which can make it delicate to directly determine the melting point.
Learn more about camphor at
https://brainly.com/question/29848323
#SPJ4
why gold is called inert metal
Answers
Gold has a heavy enough nucleus that its electrons must travel at speeds nearing the speed of light to prevent them from falling into the nucleus. This relativistic effect applies to those orbitals that have appreciable density at the nucleus, such as s and p orbitals. These relativistic electrons gain mass and as a consequence, their orbits contract. As these s and (to some degree) p orbits are contracted, the other electrons in d and f orbitals are better screened from the nucleus and their orbitals actually expand.
Since the 6s orbital with one electron is contracted, this electron is more tightly bound to the nucleus and less available for bonding with other atoms. The 4f and 5d orbitals expand, but can't be involved in bond formation since they are completely filled. This is why gold is relatively unreactive.
Hope it helps
Gold is relatively inert metal because, the one valence electron in 6s orbital is not available for bonding since it is more attracted by nucleus. The 4f and 5d orbitals are completely filled and does not involve in bonding.
What is inertness ?Some elements tends to be unreactive compared to the other by their extra stability factors. This unreactness is called inertness. The 18th group elements are all inert since they are highly stable due to completely filled electronic configuration.
Gold is 79th element in periodic table. It is a transition metal located in d-block. The electronic configuration of gold is [Xe] 4f¹⁴ 5d¹⁰ 6s¹. There is only valence electron which is in the 6s orbital.
The 4s orbital experience more nuclear pull which leads to the unavailability of the valence electron. The 4f and 5d orbitals are completely filled and does not involve in chemical bonding. That's why gold is called inert metal.
Find more on gold:
https://brainly.com/question/11405288
#SPJ2
Use the periodic table to identify the number of core electrons and the number of valence electrons in each case below.
Potassium (K): 1s22s22p63s23p64s1
18 core electrons
1 valence electrons
Iron (Fe): [Ar]4s23d6
18 core electrons
8 valence electrons
Argon (Ar): [Ne]3s23p6
10 core electrons
8 valence electrons
Magnesium (Mg): 1s22s22p63s2
10 core electrons
2 valence electrons

Answers
Yes your answers are correct.
Let's know about valence and core electrons
The electrons which are present in outer most cell of an atom called valence.The atoms which are present inert cells of an atom is called core electrons.Answer:
Yes,your answers are absolutely right/correct!
More to know:
What is valence?
Another term for valence is valency.It is the number of electrons needed to fill the outer most part of an atom.What is core electrons?
They are the electrons of an atom.They don't take part in chemical bonding.What is a species?
a group of organisms that can reproduce and have fertile offspring
the process by which inherited traits in a population change over
generations
the process by which individuals that are better adapted to their
environment are more likely to survive and reproduce
a characteristic that improves the ability of individuals in a population to
survive and reproduce

Answers
Answer:a group of organisms that can reproduce and have fertile offspring
the process by which inherited traits in a population change over
generations
Explanation:
Describe how you would separate Ultisols, mollisols,
inceptisols, and entisols bases on field morphology and associated
lab analysis?
Answers
Separating Ultisols, Mollisols, Inceptisols, and Entisols based on field morphology and associated lab analysis typically involves examining specific characteristics and performing certain tests.
Here's a general description of how you can differentiate these soil orders:
1. Ultisols:
Ultisols are typically characterized by weathering, leaching, and clay accumulation. They are often found in humid or tropical regions. To identify Ultisols, you can look for the following field morphology and perform associated lab analyses:
- Look for a well-developed soil profile with distinct horizons, such as an A horizon (topsoil), B horizon (subsoil), and often a C horizon (weathered parent material).
- Conduct a soil pH test, as Ultisols tend to be acidic (pH < 6).
- Perform chemical analyses to determine the presence of clay accumulation, iron and aluminum oxides, and leaching of bases.
2. Mollisols:
Mollisols are characterized by deep, fertile soils with a high organic matter content. They are commonly found in grassland regions. To differentiate Mollisols, consider the following:
- Look for a thick, dark, and nutrient-rich A horizon (topsoil) formed from the decomposition of organic matter.
- Conduct a soil pH test, as Mollisols are typically slightly acidic to neutral (pH around 6-7).
- Perform laboratory tests to determine high organic matter content and a high cation exchange capacity (CEC).
3. Inceptisols:
Inceptisols are soils that exhibit some degree of soil development but are not as well-developed as other orders. They can be found in various climates. To distinguish Inceptisols:
- Observe a limited soil profile development, with some horizonation but less distinct than in Ultisols or Mollisols.
- Perform laboratory analyses to determine the soil texture, pH, and mineral content.
- Look for signs of recent soil development and minimal leaching or weathering.
4. Entisols:
Entisols are soils that show minimal soil development and lack distinct horizons. They can be found in various environments. To identify Entisols:
- Observe a lack of well-defined soil horizons, often with a shallow depth.
- Conduct soil texture analysis to determine the predominant mineral composition.
- Perform laboratory tests for pH, organic matter content, and other chemical properties.
It's important to note that the identification of soil orders based on field morphology and lab analysis is a complex process that requires expertise and careful examination. Detailed field observations, soil sampling, and laboratory analyses are typically conducted by soil scientists to accurately classify and differentiate soil orders.
To know more about Ultisols
https://brainly.com/question/13267145
#SPJ11
What is the relationship of tissues to cells?
Tissues make cells.
Cells make up tissues.
Tissues are use for cell growth.
Cells help tissue grow.
Answers
A gas at 300 K and under 1 bar of pressure takes up 1.2 L of volume.
The gas is quickly compressed to 9 bars and the new temperature is measured to be 1200 K.
Use the combined gas law to calculate the new volume of the gas. Show your work!
Answers
Using the combined gas law, the volume of the gas is 1.875 L.
What is gas law?The ideal gas law tells the macroscopic properties of the gas. The particles of the gas do not repel or attract to each other.
"Volume is the space occupied by a three-dimensional object. Volume is calculated by dividing mass by density".
The combined gas law is
\(\dfrac{P_1V_1}{T_1} = \dfrac{P_2V_2}{T_2}\)
where P = pressure
V - volume
T = temperature
Given, that 300k is the temperature
The pressure is 1 bar
volume is 1.2 L
The changed pressure is 9 bars
The new temperature is 1200 K
Putting values in the formula
\(\dfrac{1 \times 1.2}{300} = \dfrac{9 \times V_2}{1200}\\\\V_2 = \dfrac{0.0075}{0.004} = 1.875 l\)
Therefore, the new volume of the gas is 1.875 L.
To learn more about combined gas law, refer to the link:
https://brainly.com/question/15204619
#SPJ1
How many neutrons does the isotope have?

Answers
Answer:
16 neutrons.
Explanation:
From the question given above, the following data were obtained:
Mass number = 30
Atomic number = 14
Neutron number =?
Next, we shall determine the number of protons present in the isotope. This can be obtained as follow:
The atomic number of an element is simply defined as the number of protons present in the atom of the element. Thus,
Atomic number = Proton number
Atomic number = 14
Therefore,
Proton number = 14
Finally, we shall determine the number of neutons present in the isotope as follow:
Mass number = 30
Proton number = 14
Neutron number =?
Mass number = Proton + Neutron
30 = 14 + Neutron
Collect like terms
Neutron = 30 – 14
Neutron = 16
Therefore, the isotope contains 16 neutrons.
Why doesn't fluorine show exceptional electronic configuration due to attaining stability??
Answers
Fluorine does not show exceptional electronic configuration because it does not have any d orbitals to move electrons to. It achieves stability by forming covalent bonds with other atoms.
Fluorine, with an atomic number of 9, has a configuration of 1s2 2s2 2p5. It is one electron short of having a full outer shell, which would make it highly stable. However, fluorine does not show exceptional electronic configuration despite this fact. This is because the exceptional electronic configuration occurs when an electron from the s orbital moves to the d orbital to achieve greater stability. However, fluorine does not have any d orbitals. Its highest energy level is the p orbital, which already has 3 electrons. Therefore, fluorine cannot attain a greater degree of stability by moving an electron to the d orbital. Instead, fluorine achieves stability by forming a covalent bond with another atom, such as hydrogen or another fluorine atom. This sharing of electrons allows fluorine to achieve a full outer shell and become highly stable.
for more questions on stability
https://brainly.com/question/2050643
#SPJ11
4. The periodic table is organized into groups and periods of elements. The point
characteristics of a certain group of elements are listed below. Which of
these elements is in this group?*
Characteristics of a Group of Elements
• Is shiny
• Is solid at room temperature
• Has atoms with two valence electrons
O A Lithium
O B. Strontium
O C. Aluminum
O D. Silicon
Answers
Answer:B
Explanation:
Arrange the following ions in order of increasing ionic radius: K+, p3-, S2-, Cl". Select one: O a. Kt
Answers
The correct arrangement of ions in increasing ionic radius is as follows:
p3- < S2- < Cl- < K+
Therefore, the correct option is:
b. p3-, S2-, Cl-, K+
In general, the ionic radius increases as you move from right to left across a period and from top to bottom within a group on the periodic table. Therefore, the arrangement of ions in increasing ionic radius is p3- < S2- < Cl- < K+.
To learn more about Ionic Radius from the given link
https://brainly.com/question/8137711
#SPJ4
Why is modern periodic table better than Mendeleev's periodic table?
Answers
Answer:
because mariah carey wrote it
Explanation:
she also made a song out of it!
Why does all matter contain thermal
energy?
A. Because particles stop moving
B. Because all matter is hot
C. Because particles can never stop moving
D. Because all matter is cold
Answers
Answer:
The answer is C. Because particles can never stop moving
Explanation:
"All matter has thermal energy, even matter that feels cold. That's because the particles of all matter are in constant motion and have kinetic energy."
indicate which factors affect the rate of a reaction. select all that apply.
Temperature
concentration k catalysts
Answers
The factors which can affect rate of reaction are temperature, concentration, and catalysts which means all of these are correct.
Reactions can be chemical or physical in nature. In physical reactions, the product formed may be reversed into their original form while in chemical reactions, the product formed is different from their original compounds called as reactants. Rate of reaction is defined at the speed at which reactants combine to form products.
As the concentration of reactants increases, rate of reaction also increases. Catalysts can help in either increasing or decreasing the rate of reaction. The catalysts which help in reducing the reaction rate are called as poison. Positive Catalysts provide additional site of binding to increase the reaction rate. If the temperature of the reaction is increased, then more easily the molecules bind or break each other and hence rate of reaction can be increased.
Learn more about rate of reaction at:
brainly.com/question/4617366
#SPJ4
which is most likely to be stable with a neutron:proton ratio of 1:1? group of answer choices nitrogen (n) bromine (br) americium (am) all of these
Answers
The most likely element to be stable with a neutron-to-proton ratio of 1:1 is nitrogen (N) and the correct option is option 1.
Stability is determined by the balance between the number of protons and neutrons in the nucleus of an atom. Nucleides that have a balanced ratio of protons to neutrons, known as the neutron-to-proton ratio, tend to be more stable. This balance is influenced by the strong nuclear force, which holds the nucleus together, and the electromagnetic repulsion between protons.
In general, nucleides with a neutron-to-proton ratio close to 1:1, known as the valley of stability, tend to be the most stable. However, stability can vary depending on the specific element and its isotopes. Nucleides that deviate significantly from the valley of stability may undergo radioactive decay, transforming into other elements or isotopes in order to achieve a more stable configuration.
Nitrogen has an atomic number of 7, meaning it has 7 protons. In order to have a neutron-to-proton ratio of 1:1, it would have 7 neutrons as well. This gives nitrogen a total of 14 nucleons (7 protons + 7 neutrons).
Both bromine (Br) and americium (Am) have atomic numbers higher than nitrogen, and their stable isotopes have neutron-to-proton ratios different from 1:1. Therefore, among the given choices, only nitrogen (N) is most likely to have a stable isotope with a neutron-to-proton ratio of 1:1.
Thus, the ideal selection is option 1.
Learn more about neutron-to-proton ratio, here:
https://brainly.com/question/30522473
#SPJ4
Which type of light causes damage to plants? (Choose one) >_<
gamma ray
X-ray
ultraviolet
violet
indigo
blue
green
yellow
orange
red
infrared
microwave
radio
Answers
the answer is ultraviolet
Answer:
microwave
Explanation:
your gonna have one crispy plant if you put it in the microwave XD
A pure yellow crystalline substane, when heated in a vacuum, releases a greenish gas and a red powder. Is the original yellow crystalline substance a compound or element?
Answers
Answer:The yellow crystalline substance is a compound. The green gas and red powder are elements.
Explanation
Elements and compounds are considered pure substances. Elements are made up of a single type of atom while compounds are composed of two or more elements. One example of a compound is the table salt (NaCl) which is made up of one atom of sodium and one atom of chlorine. The elements in the compound are combined chemically and there is a definite ratio of these elements. They are formed by a chemical reaction where chemical bonds are formed. Furthermore, the components of the compound can be separated through several means such as extraction, distillation, etc. The process of heating the yellow crystalline substance resulted in the separation of its components namely, the green gas and red powder.
