Answers
Electrons rule initially fill low-energy orbitals before moving on to higher-energy orbitals. The nitrogen atom is seen in this diagram with two electrons in the 2p subshell before the 2s subshell is fully occupied.
Which of the orbital diagram's rules is broken?One subshell in the p orbital is empty in this case, but another subshell is fully or double filled. This kind of electronic setup was against the Hund's regulation.
Which orbital diagram transgresses the Pauli exclusion rule?While pairing without single occupancy of all orbitals violates Hund's rule, two electrons with parallel spin in the same orbitals violates the Pauli exclusion principle.
To know more about Electrons rule visit:-
https://brainly.com/question/28883753
#SPJ1
Related Questions
What type of reaction is this? *
Al+HCI->AICl3 + H₂
Answers
Answer:
Balanced Reaction
hope it helps pls mark BRAINLIST
Arrange the highlighted bonds in the table below and decreasing order of polarity. That is, pick one for the most polar bond, pick two for the next most polar bond, and so on

Answers
For this exercise we need the polarity of the bonds, for that, we need the electronegativity of the elements from the bonds.
These are nonmetals, so we have covalent bonds.
The 3 of the highlighted bonds are covalent.
-----------------------------------------------------------------------
-C-C-
We calculate the polarity as a subtraction:
Polarity (-C-C-) = 0 = 2.5 - 2.5
2.5 is the electronegativity for C
-----------------------------------------------------------------------
-C-O-
Polarity (-C-O-) = 3.5 (O) - 2.5 (C) = 1.0
-----------------------------------------------------------------------
-C-F-
Polarity (-C-F-) = 4.0 (F) - 2.5 (C) = 1.5
------------------------------------------------------------------------
The electronegativities can be obtained from the Pauling scale.
Now the answer:
Decreasing order of polarity:
-C-F (the most) = 1.5 > -C-O-(the next) = 1.0 > -C-C- (the less) = 0
Male wood ducks mate only with female wood ducks. They produce male and female wood duck offspring that can mate only with other wood ducks. These facts
indicate that wood ducks:
Are inbred.
Do not mutate.
Are a species.
Lack variability
HELP
Answers
Answer:
Anyone got the answers
Explanation:
Answer: A
Explanation: just do as I say
Read each situation carefully. Write how energy transformation occurs in each situation. Write your answer on a separate sheet of paper.
1. Gerald just finish his work out at the gym. He ran at the park for 30 minutes and got very sweaty. What energy transformation took place while he was running?
2. Joshua was very hungry when he got home from the school. He decided to make some popcorn in the microwave. What energy transformation allowed Joshua's popcorn to pop?
3. Jeremy shaves every week using a battery powered shaver. What energy transformation shows how the shaver works?
4. Rica studies her lesson before going to bed at night. She makes use of a lampshade in studying her lessons. What energy transformation occurs when she plugged in the lamp shade and turned it on?
5. Dennis used to watch his favorite cartoon character every saturday using the smart television. What energy transformation occurs on the television?
\(tysm \: in \: advance\)
Answers
Answer:
1. Mechanical energy
2. Thermal energy
3. electric energy to mechanical energy
4. electrical energy to light
5. electric energy into sound energy and radiant energy
Explanation:
draw the atomic structure of 10 elements and show Electronic configuration.......
Answers
Answer:
ज
Explanation:
ूअूिkzkzkksksosawwhsusisisisisisiisisisisisisiisisisis
An object becomes positively charged when it loses electrons. O loses protons. O gains electrons. O gains neutrons.
Answers
an object becomes positively charged when it LOSES ELECTRONS.
* protons are positive, so losing protons means that it would be negatively charged.
* gaining electrons would make it more negatively charged.
* gaining neutrons changes nothing because neutrons are neutral.
What is the molarity of a solution that is made by mixing 35.5 g of Ba(OH)2 in 325 mL of solution?
Answers
Given :
Mass of Ba(OH)₂ , m = 35.5 g.
Volume of mixture, V = 325 mL = 0.325 L.
To Find :
The molarity of a solution.
Solution :
We know, molarity of a solution is given by :
\(M = \dfrac{Given \ Weight}{Molecular\ Mass\times Volume( L ) }\)
We know, molecular mass of Ba(OH)₂ is given by :
M.M. = 171 g/mol
Putting all these values in given equation, we get :
\(M = \dfrac{35.5}{171\times 0.325}\ M\\\\M = 0.64\ M\)
Hence, this is the required solution.
Carbon dioxide is an example of a greenhouse gas. Levels of carbon dioxide are increasing in the atmosphere. How are increasing levels of carbon dioxide affecting the atmosphere?
Select the two correct awnsers.
1.less water is evaporating from the oceans
2. Earthquakes and volcanic eruptions are becoming more frequent
3.patterns of rain and snow are changing
4.ice caps are becoming thicker and wider at the North Pole and South Pole
5.oceans waters are becoming warmer
Answers
Global warming brought on by rising carbon dioxide concentrations in the atmosphere is changing weather patterns and ocean temperatures. Changes in precipitation patterns are being brought on by the warming of the atmosphere.
Why does carbon dioxide serve as a representative greenhouse gas?Because it is one of the gases in the atmosphere that causes the greenhouse effect, which causes the Earth to warm, carbon dioxide is known as a greenhouse gas. Long-wavelength infrared radiation (heat) from the Earth is absorbed by carbon dioxide molecules in the atmosphere, and some of it is then radiated back downward.
What impact does an increase in atmospheric carbon dioxide have?Similarly, as air temperatures rise in response to rising carbon dioxide concentrations, more water vapor escapes into the atmosphere—which then amplifies greenhouse heating
To know more about greenhouse gas visit:-
brainly.com/question/4509458
#SPJ9
how can you use evaporation to separate water from food coloring
Answers
Explaination: hope this helps
What product(s) would be formed when these are the reactants? C5H12 + O2 (limited)
Answers
Answer:
Carbon dioxide and water I believe because it is a combustion reaction
KCIO3 -> KCI + 02
How many moles of KCI are produced if 6743 grams of KCIO3 decomposes?
Answers
55.03 moles of KCI are produced when 6743 grams of \(KClO_{3}\) decomposes
To determine the number of moles of KCl produced when 6743 grams of \(KClO_{3}\) decomposes, we need to use the concept of molar mass and the balanced chemical equation.
First, let's calculate the molar mass of \(KClO_{3}\)
The molar mass of potassium (K) is approximately 39.10 g/mol.
The molar mass of chlorine (Cl) is approximately 35.45 g/mol.
The molar mass of oxygen (O) is approximately 16.00 g/mol.
So, the molar mass of \(KClO_{3}\) is:
(39.10 g/mol) + (35.45 g/mol) + (3 * 16.00 g/mol) = 122.55 g/mol.
Now, we need to calculate the number of moles of \(KClO_{3}\):
Number of moles = Mass / Molar mass
Number of moles = 6743 g / 122.55 g/mol = 55.03 mol.
According to the balanced chemical equation:
2\(KClO_{3}\) -> 2 KCl + 3 O2,
we can see that for every 2 moles of \(KClO_{3}\), we obtain 2 moles of KCl.
Therefore, the number of moles of KCl produced will be equal to the number of moles of \(KClO_{3}\) since the ratio is 1:1. Thus, 55.03 moles of KCl will be produced.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ11
how do you fight off ADHD medication
Answers
Answer:A medication break can ease side effects. A lack of appetite, weight loss, sleep troubles, headaches, and stomach pain are common side effects of ADHD medication.
Explanation: It may boost your child’s growth. Some ADHD medications can slow a child’s growth in height, especially during the first 2 years of taking it. While height delays are temporary and kids typically catch up later, going off medication may lead to fewer growth delays.
It won’t hurt your child. Taking a child off ADHD medication may cause their ADHD symptoms to reappear. But it won’t make them sick or cause other side effects.
Chemistry problems
1. 1.5 moles of potassium sulfate (K SO4) were dissolved in 1000 grams of water (H2O). Find the % and Cm.
2. 10 grams of sulfuric acid (H2SO4) was added to 500 ml of 10% solution of potassium hydroxide (KOH) with a density of 1.1 g/ml. Find the mass of potassium sulfate (K SO4) formed.
3. Find the mass of the salt formed by the reaction of 7.3 grams of hydrochloric acid (HCl) with 5.6 liters (5600 ml) of ammonia (NH3).
4. Find the volume of hydrogen gas (H2) produced by the reaction of 13 grams of zinc with a solution containing 30 grams of sulfuric acid (H2SO4).
5. How much of the concentrated original solution (70%) of acetic acid is needed to prepare 500 grams of 3% (percentage solution)?
Answers
1. The % concentration is 20.7% and the molar concentration, Cm, is 1.5 M.
2. 7.8 grams of potassium sulfate will be formed.
3. 10.7 grams of ammonium chloride will be formed.
4. The volume of hydrogen gas that will be produced is 3.86 liters.
5. 21.43 grams of the 70% acetic acid is needed to prepare 500 grams of 3% acetic acid solution.
What is the percentage concentration?1. Mass of potassium sulfate = 1.5 moles * (174.26 g/mol) = 261.39 g
Mass of water (H₂O) = 1000 g
% = (mass of solute/mass of solution) x 100
% = (261.39 g / (261.39 g + 1000 g)) x 100
% ≈ 20.7%
Cm = moles of solute / volume of solution
Moles of potassium sulfate (K2SO4) = 1.5 moles
Volume of water (H2O) = 1000 g / (density of water) = 1000 g / 1 g/mL = 1000 mL = 1 L
Cm = 1.5 moles / 1 L
Cm = 1.5 M
2. The balanced equation for the reaction is:
H₂SO₄ + 2 KOH → K₂SO₄ + 2 H₂O
Molar mass of sulfuric acid (H₂SO₄) = 98.09 g/mol
Moles of sulfuric acid = 10 g / 98.09 g/mol
Moles of sulfuric acid = 0.102 mol
Based on the mole ratio of the reaction, 0.102 moles of sulfuric acid will react to form 0.102 moles of potassium sulfate.
Molar mass of potassium sulfate = 174.26 g/mol
Mass of potassium sulfate = 0.102 mol x 174.26 g/mol
Mass of potassium sulfate ≈ 17.8 g
3. The balanced equation for the reaction is:
HCl + NH₃ → NH₄ClMolar mass of hydrochloric acid (HCl) = 36.46 g/mol
Moles of hydrochloric acid (HCl) = 7.3 g / 36.46 g/mol
Moles of hydrochloric acid ≈ 0.2 mol
Based on the mole ratio of the reaction, 0.2 moles of hydrochloric acid will react to form 0.2 moles of ammonium chloride.
Molar mass of ammonium chloride (NH₄Cl) = 53.49 g/mol
Mass of ammonium chloride = 0.2 mol x 53.49 g/mol
Mass of ammonium chloride ≈ 10.7 g
4. The balanced equation for the reaction is:
Zn + H₂SO₄ → ZnSO₄ + H₂Molar mass of zinc (Zn) = 65.38 g/mol
Moles of zinc = 13 g / 65.38 g/mol
Moles of zinc ≈ 0.199 mol
Based on the mole ratio of the reaction, 0.199 moles of zinc will react to produce 0.199 moles of hydrogen gas.
Volume of sulfuric acid = 30 g / (density of H₂SO₄ )
The density of H₂SO₄ is 1.84 g/mL
Volume of sulfuric acid = 30 g / 1.84 g/mL
Volume of sulfuric acid ≈ 16.3 mL or 0.0163 L
Using the ideal gas law, the volume of hydrogen gas produced will be:
V = nRT / P
V = (0.199 mol)(0.0821 L·atm/(mol·K))(273 K) / (1 atm)
V ≈ 3.86 L
5. Assuming that the concentrated original solution of acetic acid is 100% acetic acid (CH₃COOH).
Mass of acetic acid = 500 g x (3/100) = 15 g
The concentrated original solution, however, is 70% acetic acid.
70% acetic acid (mass) = 100% acetic acid (unknown mass)
0.7 * (unknown mass) = 15 g
Solving for the unknown mass:
unknown mass = 15 g / 0.7
unknown mass ≈ 21.43 g
Learn more about percentage concentration at: https://brainly.com/question/18761928
#SPJ1
which element has the electrons configuration 1s22s22p63s23p64s23d104p2
Answers
The element with the electron configuration 1s22s22p63s23p64s23d104p2 is Silicon (Si).
Explanation:
The electron configuration of an element describes the arrangement of electrons in its atoms. The numbers and letters in the configuration represent the energy levels (n), sublevels (s, p, d, f), and the number of electrons in each sublevel.
In this case, the electron configuration of the element is:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Breaking this down, we can see that the element has:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 6 electrons in the 2p sublevel
- 2 electrons in the 3s sublevel
- 6 electrons in the 3p sublevel
- 2 electrons in the 4s sublevel
- 10 electrons in the 3d sublevel
- 2 electrons in the 4p sublevel
Based on the number of electrons in the outermost energy level (valence electrons), we can determine that this element is in group 14 of the periodic table. Looking at the periodic table, we can see that the
2. A restaurant offers a $19.99 three course meal special that lets you choose an appetizer,
an entree, and a dessert. There are 8 appetizers, 12 entrees, and 6 desserts from which
to choose. How many different meals are available?
Answers
There are 576 different ways to choose a meal.
Promotional offers. A promotional offer is a specific proposition to clients that specifies a reward and patron behavioral standards for earning a reward. the recognition and trouble of the praise are captured thru a retail transaction, purchaser order, rebate claim, rebate redemption, or different patron interplay. a discount is a difference between the unique charge and the lower charge it's far being bought at. a proposal is a deal wherein a product is normally bought at a reduction.
Calculation:-
There are 8 appetizers, 12 entrees, and 6 desserts.
Number of choices = 8 × 12 × 6
= 576
Learn more about different meals here:-https://brainly.com/question/8880115
#SPJ1
100.0 degrees C a sample of 750mL gas is found. If the temperature of the gas is increased by 300 degrees, what will be the new volume gas
Answers
100.0 degrees Celsius = 100.0 + 273 = 373.0 K
\(\frac{750}{373.0}=\frac{V_{2}}{673.0} \\ \\ V_{2}=\frac{(673.0)(750)}{373.0} \approx \boxed{1350 \text{ mL}}\)
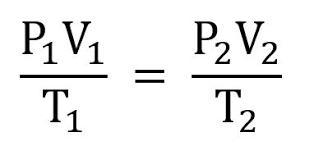
Determine the percent water in MgSO4*7H20?
Answers
Answer:
\(\% H_2O=51.2\%\)
Explanation:
Hello!
In this case, since the percent water is computed by dividing the amount of water by the total mass of the hydrate; we infer we first need the molar mass of water and that of the hydrate as shown below:
\(MM_{MgSO_4* 7H_2O}=120.36 g/mol+7*18.02g/mol\\\\MM_{MgSO_4* 7H_2O}=246.5g/mol\)
Thus, the percent water is:
\(\% H_2O=\frac{7*MM_{H_2O}}{MM_{MgSO_4* 7H_2O}} *100\%\\\\\)
So we plug in to obtain:
\(\% H_2O=\frac{7*18.02}{246.5} *100\%\\\\\% H_2O=51.2\%\)
Best regards!
Topic 2: The Concepts
3. In ionic equations, mass and charge must be balanced on both sides to form a
proper reaction. Why is this the case? If you have a combination of solutions that
forms a precipitate, what factor determines the amount of precipitate that will
form? (Hint: If you have more of one ion than another as the precipitate is
forming, what will happen?)
Answers
The mass and charge must be balanced so that the compound formed is neutral. The limiting reactant determines the amount of the precipitate that can be formed.
What is ionic equation?When we talk about an ionic equation, we are talking about the kind of equation that is able to show the ions that are able to undergo a change in a given reaction. We know that in many cases the ionic reaction would lead to the formation of a precipitate and this is the solid product that is obtained when we happen to mix two solutions that are known to be aqueous solutions.
Having made all that introduction, we know that a compound is said to be neutral when the charges in the species that form the compound could be seen as being balanced. This is the reason why the mass and charge must be balanced when we are dealing with an ionic reaction equation.
The factor that determines the amount of precipitate that would form is the limiting reactant of the process.
Learn more about precipitate:https://brainly.com/question/16950193
#SPJ1
For the general gas phase reaction 2 A + 3 B ↔ 2 C the equalibrium constant is 5.00. Calculate the equilibrium quotient based on the following initial concentrations and predict whether the system with shift left or shift right.
Report your answer to 3 significant figures, not scientific notation, using the format "1.23 left" or "9.87 right".
A0 = 0.293 M
B0 = 2.1 M
C0 = 0.036 M
Answers
The equilibrium quotient based on the following initial concentrations and predict whether the system with shift left or shift right is 3.10 x 10⁻⁴ right
Gas Phase Reactions :There are two types of gas-phase reactions: intramolecular and intermolecular. Precursor molecules are broken down into activated species in intramolecular reactions, which are then used in the CVD process.
How can you calculate and determine the shift?Qc = [C] = equilibrium quotient ² / [A]² [B]³
Qc = (0.0383)² / (0.219)² (4.62) (4.62)³
Qc = 3.10 x 10⁻⁴
Kc = 5.00
Kc > Qc . so shifts to right
What is the formula for the gas-phase reaction?We use the ideal gas equation PV=nRT to account for these conditions, where P is the pressure in atmosphere (atm), V is the volume in liters (L), n is the number of moles, and R is the gas constant with a value of 08206 L atm mol-1 K-1, where T is the temperature in kelvin (K) and L is the atm.
Learn more gas phase reaction :
brainly.com/question/14789122?
#SPJ1
Cobalt-60 has a half-life of about 5.26 years. If a sample has an initial mass of 4.48 grams and undergoes decay until it has a mass of 0.140 grams, then how many half-lives passed?
a)4 half-lifes
b) 3 half-lifes
c) 5 half-lifes
Answers
If a sample has an initial mass of 4.48 grams and undergoes decay until it has a mass of 0.140 grams, then option b 3 half-lifes passed
The half-life of a substance is the amount of time it takes for half of the initial sample to decay. For cobalt-60, the half-life is 5.26 years. This means that if we start with 4.48 grams of cobalt-60, half of that (2.24 grams) will remain after 5.26 years.
The remaining 2.24 grams will then decay until half of that (1.12 grams) remains after another 5.26 years. Continuing this pattern, we can see that after 3 half-lives (15.78 years), we will be left with 0.140 grams of cobalt-60. Therefore, the sample underwent decay for 3 half-lives.
Half-life, in chemistry, is the time required for half of the original number of radioactive atoms in a sample to decay. The half-life of a radioactive substance is a measure of the stability of the nucleus of the atom.
Learn more about half-lives:
https://brainly.com/question/24710827
https://brainly.com/question/16980763
The question is in the picture

Answers
Charles's law of gases states that the density of an ideal gas is inversely proportional to its temperature at constant pressure.
The equation is as follows;
Va/Ta = Vb/Tb
Where;
Va and Ta = initial volume and temperature respectivelyVb and Tb = final volume and temperature respectively0.67/362 = 1.12/Tb
0.00185Tb = 1.12
Tb = 605.41K
This temperature in °C is 605.41 - 273 = 332°C
Learn more about Charles's law at: https://brainly.com/question/16927784
#SPJ1
Write a short essay about life in the Han Dynasty, comparing it to life today. Make sure to include key features:
-Family
-Government
-Social Structure
-Religion
-Trade
Answers
Answer:
Life in the Han Dynasty (206 BCE - 220 CE) differed significantly from today in family, government, social structure, religion, and trade. For example, the Han Dynasty emphasized a patriarchal family structure, where the eldest male held authority, and filial piety was highly valued. In contrast, contemporary societies embrace more egalitarian family dynamics with shared decision-making.
The government system of the Han Dynasty relied on a centralized bureaucracy and emphasized meritocracy, while modern societies often adopted democratic systems. Socially, the Han Dynasty followed a hierarchical model influenced by Confucian principles, whereas contemporary societies strive for greater equality and social mobility.
Religion in the Han Dynasty combined Confucianism, Taoism, and Buddhism, whereas modern societies exhibit diverse religious beliefs. Lastly, trade in the Han Dynasty thrived along the Silk Road, while modern trade was globally interconnected and facilitated by technological advancements. These differences highlight the evolution of society over time.
Explanation:
Describe in your own words what is an air front is
Answers
Answer:
A front is a weather system that is the boundary separating two different types of air. One type of air is usually denser than the other, with different temperatures and different levels of humidity.
The heat of combustion of CH4 is 890.4 KJ/mol, and the heat capacity of H20 is 75.2 J/molK. Find the volume of methane measured at 298K and 1.00 atm required to convert 1.00L of water at 298 K to water vapor at 373K.
Answers
Q methane = Q water
Q = mcΔt
mass water = V x ρ = 1 L x 1 kg/L = 1 kg
298 K to 373K = 25 °C to 100 °C
c water = 75.2 J/mol K = 4.2 J / g °C = 4200 J/kg °C
Q water = 1 x 4200 x (100 - 25)
Q water = 315000 J
Q methane = Q water = 315000 J = 315 KJ
n (mol) methane = ΔH/Q = 890.4 / 315 = 2.83
PV = nRT
V = nRT/P
V = 2.83 x 0.082 x 298/1
V = 69.15 L
What is the ratio between the numbers 30 and 90
Answers
1:3
you can divide it
a. Using the Born-Mayer Equation, calculate the lattice enthalpy for sphalerite
(zinc blende), ZnS. You must look up the appropriate parameters for the equation.
b. Using the Born-Mayer Equation, calculate the lattice enthalpy for wurtzite, ZnS. You must
look up the appropriate parameters for the equation.
c. Which is thermodynamically stable at ambient conditions (25 °C, 1 bar)? Find a reference
with the T and P phase diagram for ZnS. Submit the pdf of the reference with your file . Also,
compare your answer to the standard enthalpies of formation for wurtzite compared to sphalerite.
Answers
ΔLatticeU = ΔLatticeH – pΔVm is the lattice energy of wurtzite. Ionic compounds often have flat surfaces that meet at distinctive angles and are stiff, brittle, crystalline materials.
Remember that when a metal reacts with a nonmetal, often an ionic compound results from the transfer of electrons form the metal (the reductant) towards the nonmetal (the oxidant). Ionic compounds often have flat surfaces that meet at distinctive angles and are stiff, brittle, crystalline materials. They melt at rather high temperatures and are not easily distorted. ΔLatticeU = ΔLatticeH – pΔVm is the lattice energy of wurtzite.
To know more about lattice energy, here:
https://brainly.com/question/29735933
#SPJ1
during a baseball game the sound of the bat hitting the ball
Answers
During a baseball game, the sound of the bat hitting the ball can be heard in most parts of the stadium. That sound is weaker at greater distances. The cause of this phenomenon is inverse-square law.
What is sound?Sound is a physical disturbance from an equilibrium condition that travels via an elastoplastic medium. A completely subjective definition of sound, as perceived by the ear, is also viable, but it is not especially informative and is overly limited, because it is important to speak of noises which can be heard by the auditory system, such as those produced by dog whistles or sonar technology.
The inverse-square law, that states that the strength of a sound wave is inversely related to the square of the distance to the source, is the cause of this phenomena. The strength of such sound wave reduces as the distance to the source rises.
Therefore, the cause of this phenomenon is inverse-square law.
To learn more about sound, here:
https://brainly.com/question/733324
#SPJ1
An aqueous KNO3 solution is made using 77.2 g
of KNO3 diluted to a total solution volume of 2.06 L. (Assume a density of 1.05 g/mL
for the solution.) Calculate the mass percent of the solution.
Answers
An aqueous KNO\(_3\) solution is made using 77.2 g of KNO\(_3\) diluted to a total solution volume of 2.06 L. 3.33% is the mass percent of the solution.
What is mass percent?Mass percent is the means to indicate a concentration. Furthermore, it describes the element in a certain composition. A mass percentage can be used to understand the solution's composition. It displays the amount of solute in a certain amount of solution. The solute's concentration is specified in terms of mass or moles.
In order to represent the mass percent of a solution, the kilograms of solute are split by the kilograms of solution, and the result is multiplied by 100.
Number of moles of KNO\(_3\) = mass/molar mass = 77.2 /101 g= 0.72 moles
Molarity = Number of moles / volume = 0.72 / 2.06 = 0.36 mol/L
molality = Number of moles of solute/Mass of solution in kilograms
mass of solution = 1.05 g/mL × 2000 mL = 21000 g=2.1Kg
Mass percent of solution = mass of solute/mass of solution × 100
Mass percent of solution = 77.2 g/ (77.2 g + 2100 g) × 100
= 3.33%
Therefore, 3.33% is the mass percent of the solution.
To learn more about mass percent, here:
https://brainly.com/question/5394922
#SPJ1
2. a. Draw and label an energy diagram similar to the one shown in the sample problem for a reaction in which E= 125 kJ/mol and E' = 86 kJ/mol. Place the reactants at energy level zero. b. Calculate the values of AE, forward and AEreverse. c. Is this reaction endothermic or exothermic? Explain your answer.
3. a. Draw and label an energy diagram for a reaction in which E= 154 kJ/mol and AE136 kJ/mol. b. Calculate the activation energy, E, for the reverse reaction.

Answers
The reaction is endothermic since the energy level of the products have are higher than that of the reactants.
What are the values of AE and E?The activation energy (AE) is the energy difference between the reactants and the transition state.
The change in energy E and the energy difference between the reactants and the products
The data given is as follows:
Reactants: 0 kJ/mol
AE forward 125 kJ/mol
AE reverse: 86 kJ/mol
Products: 39 kJ/mol
The values of ΔE forward and ΔE reverse are as follows:
ΔE forward = (39 - 0) kJ/mol
ΔE forward = +39 kJ/mol
ΔE reverse = (0 - 39) kJ/mol
ΔE reverse = -39 kJ/mol
3. Given that Ea = 154 kJ/mol and ΔE = 136 kJ/mol
AE reverse = ΔE - AE forward
E = 136 kJ/mol - 154 kJ/mol
E = -18 kJ/mol
Learn more about activation energy at: https://brainly.com/question/1380484
#SPJ1

What is magma?
solid rock with a fine texture
cooled rock with large crystals
molten rock below Earth’s surface
flowing rock above Earth’s surface
Answers
Answer:
C. molten rock below Earth’s surface
Explanation:
