Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. MnO4 (aq) → MnO2(s) 4- 13 3- 12 2+ 13+ 4+ ] 2. 1 ] 4 1. 5 6 7 8 o 1 02 ó 3 . O 06 U O: O, 8 0. _ 10 + ( ) 11 (s) (1) D (g) (aq) H e- H+ H3O+ Mn OH H20
Answers
The half-reaction for the reduction of permanganate ion (\(MnO_4^-\)) to manganese dioxide (\(MnO_2\)) in acidic solution is:
\(MnO_4^- (aq) + 8H^+ (aq) + 5e^-\) →\(MnO_2 (s) + 4H_2O (l)\)
The balanced half-reaction shows that eight hydrogen ions (\(H^+\)) and five electrons (\(e^-\)) are required to reduce one permanganate ion (\(MnO_4^-\)) to one manganese dioxide molecule (\(MnO_2\)). The oxidation state of manganese decreases from +7 in \(MnO_4^-\) to +4 in \(MnO_2\).
Note that the state of water (\(H_2O\\\)) is changed to \(H^+\) and \(OH^-\) in the presence of acid (\(H^+\)), resulting in the formation of \(H_2O(l)\) instead of \(H_2O(aq)\) in the balanced half-reaction.
For more question on half-reaction click on
https://brainly.com/question/23029816
#SPJ11
Related Questions
If you add energy to water that is at 100 degrees Celsius and 1 atm pressure, the temperature will?A. Go downB. Stay the sameC. Rise
Answers
ANSWER
Temperature will remain the same
EXPLANATION
When water reaches it boiling point at 100 degrees Celcius, the molecules of water will start moving independently and collide with each other.
At 100 degrees Celcius, it has attained an equilibrium between the liquid and vapor phase. Therefore, when energy is added to it, the temperature will remain the same and there will be a phase change.
Hence, the temperature will remain the same.
when scientist say a therory can be proven what are they acually saying
Answers
Answer:
it means that things people come up with can be tested and they can turn it into the truth or in another sense figure out if its true or not
Explanation:they can figure out if its real or not and or turn it into the facts from what people make up and or think so and so is this or that and they can find out the truth
Nate and Clara are drawing pictures with markers. There are 8 markers in a set. Nate has 9 markers and Clara has 7. What can Nate and Clara do so that each of them has a full set?
Answers
9-1=8
7+1=8
nitial concentration of hc2h3o2 initial concentration of nac2h3o2 calculated ph change in ph make sure to include u
Answers
To calculate the pH change of a solution with an initial concentration of HC2H3O2 (acetic acid) and NaC2H3O2 (sodium acetate),
follow these steps:
1. Identify the initial concentrations of HC2H3O2 and NaC2H3O2 given in the problem.
2. Determine the Ka value of HC2H3O2 (acetic acid) from a reference source, which is 1.8 x 10^(-5).
3. Calculate the initial pH of the solution using the formula: pH = -log([H+]), where [H+] is the concentration of hydrogen ions in the solution.
4. Since NaC2H3O2 is the conjugate base of HC2H3O2, use the Henderson-Hasselbalch equation to calculate the new pH after adding NaC2H3O2: pH = pKa + log([A-]/[HA]), where pKa = -log(Ka), [A-] is the concentration of the conjugate base (NaC2H3O2), and [HA] is the concentration of the weak acid (HC2H3O2).
5. Calculate the pH change by subtracting the initial pH from the new pH.
By following these steps, you can determine the pH change when the initial concentrations of HC2H3O2 and NaC2H3O2 are given.
To learn more about ph change, visit:
https://brainly.com/question/29484611
#SPJ11
Why is it easier to remove an electron from potassium than sodium?
Answers
The diagram below shows the different phase transitions that occur in matter.
0000
Solid
2345
Liquid
Gas
Which arrow would most likely represent the phase change that involves the same amount of energy as arrow 1?
02
6

Answers
The phase diagram represents the different phase transitions that occur in matter. The arrow labeled "1" represents the transition from a solid to a liquid state, which is commonly known as melting or fusion.
When a substance undergoes melting, it absorbs a specific amount of energy known as the latent heat of fusion. To identify the arrow that most likely represents a phase change involving the same amount of energy as arrow 1, we need to consider the specific phase transitions and their associated energy changes. The phase transition directly opposite to melting on the phase diagram is the transition from a liquid to a solid state, known as freezing or solidification. This transition involves the release of the same amount of energy that was absorbed during melting.
Hence, the arrow that most likely represents the phase change involving the same amount of energy as arrow 1 is arrow "6," which signifies the transition from a liquid to a solid state. Both melting and freezing involve the same amount of energy exchange, as they are reversible processes occurring at the same temperature.
For more questions on fusion, click on:
https://brainly.com/question/17870368
#SPJ8
how many protons electrons and neutrons does the following isotopes contain 1H+
Answers
Answer:
H+ contains 1 proton, 0 neutrons, 0 electrons.
Explanation:
Fresh air contains approximately 300 ppm CO2, whereas the breath of an intoxicated person contains about 3 percent CO2. The amount of CO2 in the breath of an intoxicated person is ________ times the amount of CO2 in fresh air.
Answers
Fresh air contains approximately 300 ppm CO2, whereas the breath of an intoxicated person contains about 3 percent CO2. The amount of CO2 in the breath of an intoxicated person is 100 times the amount of CO2 in fresh air.
How many times the amount of CO2 is in the breath of an intoxicated person as compared to fresh air? The amount of CO2 in the breath of an intoxicated person is 100 times the amount of CO2 in fresh air. Key Points Carbon dioxide concentration in fresh air is approximately 300 ppm. The carbon dioxide concentration in the breath of an intoxicated person is roughly 3 percent of the total volume of exhaled air. Calculation: We are given that the concentration of carbon dioxide in fresh air is roughly 300 ppm.
We are also told that the concentration of carbon dioxide in the breath of an intoxicated person is roughly 3 percent of the total volume of exhaled air. To calculate the answer, we can use the fact that 1 percent is the same as 1/100 or 0.01. As a result, we can convert the percent value to a decimal by dividing by 100.Therefore, 3 percent of the total volume of exhaled air is 0.03. Next, we can compare this value to the concentration of carbon dioxide in fresh air, which is roughly 300 ppm.
To do so, we will divide the concentration of CO2 in the breath of an intoxicated person by the concentration of CO2 in fresh air:0.03 / (300 ppm) = 0.0001 or 1/10000.1/10000 is the same as 1/1000 x 1/10. So, 1/10 is the same as 10/100. Therefore,1/1000 x 10/100 = 10/1,000= 1/100 times or0.1 times
In conclusion, the amount of CO2 in the breath of an intoxicated person is 100 times the amount of CO2 in fresh air.
To know more about Fresh air refer here:
https://brainly.com/question/31783892#
#SPJ11
In which of the following statements best describes the sun's
convective zone?
The layer of the sun where nuclear
fusion occurs and gives the sun its
energy
The layer of the sun where hot plasma
rises, then falls as it cools near the
surface.
O The layer of the sun seen during a
solar eclipse.
The layer of the sun that radiates
energy outwards from the sun's core.
Answers
Answer:
The answer is the layer of the sun that radiates energy outwards from the sun core
Explanation:
the reason why that is becuase the convection zone is a layers which is unstable to convection. And energy is primarily or partially transported by convection
Answer:
D
Explanation:
Help! Please the due date is soon

Answers
Answer: 68 and liquid
Explanation:
68 because the room temperature is balanced with the fish bowl and liquid because milk can flow and doesn’t have a fixed volume
If Frank creates 20 waves every 10 seconds, then how many waves does he create EVERY SECOND?
Answers
Answer:
2 waves
Explanation:
If Frank creates 20 waves every second then the way you solve is turn it into a proportion 20 waves/10 secs = x/1 sec which then turns into the equation of 10x=20 where x=2 hence Franks creates 2 waves every second.
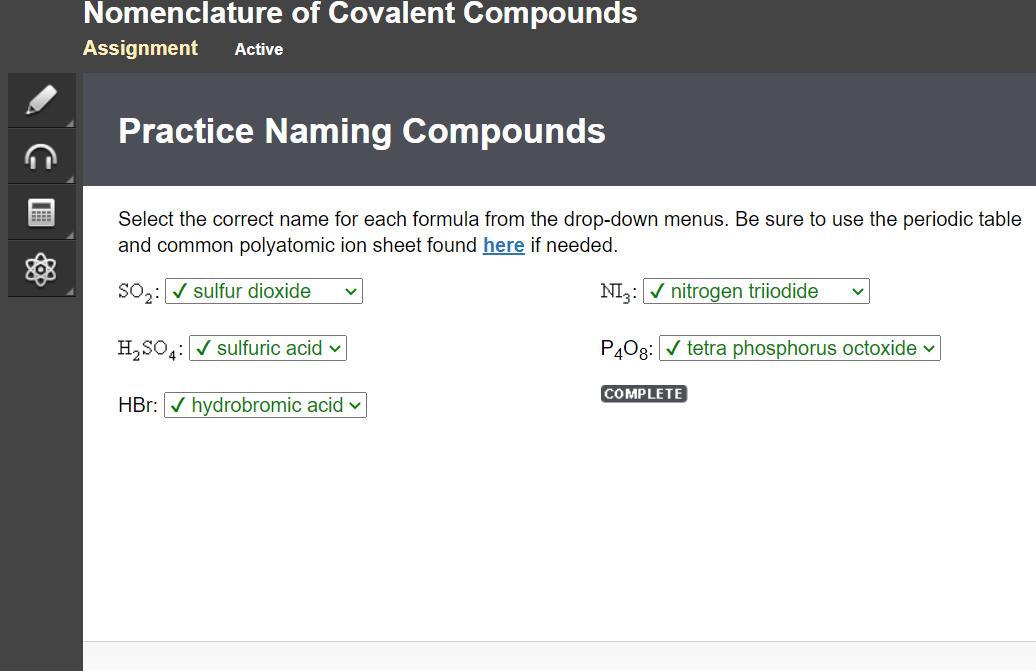
Select the correct name for each formula from the drop-down menus. Be sure to use the periodic table
and common polyatomic ion sheet found here if needed.
Answers
Answer:
here are the answers edge2020
Explanation:

Answer:
Here is 2/5 and the next slide answers too!
Explanation:
Hope it helps!!

Which of the following is NOT a function of the skeletal system?
moving blood through your body
protecting soft body parts
supporting your body
providing a place for muscles to attach
Answers
Answer:
Moving blood through your body
Explanation:
Thats the job of vascular system IE heart arteries and veins.
A student investigates the reaction between sodium hydroxide solution and dilute sulfuric acid.
He does a titration to find the concentration of the sulfuric acid.
(a) Give the colour change of the methyl orange indicator at the end-point.
Answers
Explanation:
the colour change will be from orange to pink
The color change of the methyl orange indicator at the end-point will show orange to pink.
What is indicator?In an acidic or basic medium, an indicator is just a material that changes color. Indicators are termed indicators because they show one color in an acidic media and different colors in a basic medium. Indicators come in a variety of shapes and sizes: Natural indicators are indicators acquired from natural sources.
It is a just a acid base reaction in which methyl orange acts as a indicator.
In this reaction, when sodium hydroxide solution and dilute sulfuric acid are reacts with each other then after using( indicator)methyl orange the color will be change form orange to pink which indicates the nature of solution will be acidic.
To know more about indicator.
https://brainly.com/question/12489874.
#SPJ2
How can we write a chemical reaction that explains what happens during a chemical change?
Answers
Answer: When we represent a chemical reaction in terms of words, we write a word equation. For example, when hydrogen gas reacts with oxygen gas to form water, we can write a word equation for the reaction as follows: hydrogen + oxygen→ water To the left of the arrow, we have the 'before' situation.
Explanation:
Instructions: In this virtual lab, you will use a virtual spectrometer to analyze astronomical bodies in space. Record your hypothesis and spectrometric results in the lab report below. You will submit your completed report to your instructor.
Answers
The main function of using a virtual spectrometer in order to analyze astronomical bodies in space is to find the chemical composition of planets and stars.
What is a Virtual Spectrometer?This refers to the instrument that is used to observe the color separation of light in a controlled experiment.
Hence, we can see that another reason for the use of a virtual spectrometer to make an analysis of astronomical bodies is to indicate the speed and direction of a star or galaxy as it spreads incoming beams of light into different spectrums.
Read more about virtual spectrometers here:
https://brainly.com/question/21578855
#SPJ1
Jacinta wrote the following passage about chemical changes.
A chemical change is a change that results in one or more new substances with different properties. When a chemical change occurs, the new substance can give off an odor, such as rotting fruit or cookies baking in the oven. Chemical changes can cause color changes, such as iron rusting and turning a reddish-brown color. When wood is burned or ingredients are mixed for baking a cake, a new substance is made.
Choose the best statement that Jacinta can add to her passage.
Chemical changes, such as rotting fruit and burning wood, are affected by temperature. More heat and energy causes a faster chemical change.
Wood burning and fruit rotting creates new substances through a chemical change that is affected by sound energy.
Rotting fruit and burning wood is a chemical change that occurs because there is a decrease in temperature. Less heat causes a faster chemical change.
When a chemical change occurs, the new substance that is created has properties similar to the original substance.
Answers
which five atoms tend to form double of triple bonds? explain why these atoms form double and triple bonds.
Answers
Answer:
The formation of double and triple bonds is not as widespread among the atoms of the periodic table as one might expect. At least one of the atoms involved in a multiple bond is almost always C, N, or O, and in most cases both atoms are members of this trio
Covalent bonding occurs when electrons are shared between atoms. Double and triple covalent bonds occur when four or six electrons are shared between two atoms, and they are indicated in Lewis structures by drawing two or three lines connecting one atom to another.
Explanation:
How does what you learned in this investigation help you explain why chefs measure the amount of ingredients they need before preparing foods?
Answers
Chefs measure the number of ingredients they need before preparing foods for accuracy, consistency, and balancing flavors.
Measurements ensure accuracy and consistency in recipes. Cooking is a precise process, and precise measurements of ingredients are crucial for achieving the desired taste, texture, and overall outcome of a dish. By measuring ingredients, chefs can replicate their recipes consistently, ensuring that each dish turns out as intended.
Certain ingredients, such as spices, seasonings, and acids, can greatly impact the taste of a dish. By carefully measuring these ingredients, chefs can maintain a precise balance of flavors.
Learn more about accuracy, here:
https://brainly.com/question/13099041
#SPJ1
How many Valence Electrons do Lithium has ??
Answers
Answer:
one valence electron
Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons.
- Lumen Learning
Answer:
one
Explanation:
because lithium is the third element with a total of 3 electrons.
Competition of mineral formation! Dolomite, CaMg(CO3)2, is another common carbonate rock, with logK=−17.09 and the reaction as follow: CaMg(CO3)2⇌Ca2++Mg2++2CO32− In the water sample of question lb, if [Mg2+]∼0.10mmolL−1, which mineral (calcite or dolomite) would form first? Hint: Calculate the Q/K ratios for each mineral. This ratio is also commonly referred to as the saturation index; the mineral with higher SI will be more likely to precipitate first.
Answers
By performing the necessary calculations and comparing the Q/K ratios, we can determine whether calcite or dolomite would form first in the given water sample with [Mg2+]∼0.10 mmol/L.
To determine which mineral, calcite or dolomite, would form first in the given water sample with [Mg2+]∼0.10 mmol/L, we need to calculate the saturation index (SI) for each mineral by comparing the Q/K ratios. The mineral with the higher SI will be more likely to precipitate first.
The saturation index (SI) is calculated by comparing the ion activity product (Q) with the equilibrium constant (K) for a particular mineral. In this case, we have the equilibrium reaction: CaMg(CO3)2⇌Ca2++Mg2++2CO32−.
For calcite, the Q/K ratio can be calculated using the concentration of Ca2+ and CO32− ions in the water sample. Since dolomite contains both Ca2+ and Mg2+ ions, we need to consider the concentration of Mg2+ as well.
By comparing the Q/K ratios for calcite and dolomite, we can determine which mineral has a higher saturation index (SI). The mineral with the higher SI will be more likely to precipitate first.
Learn more about equilibrium constant here:
https://brainly.com/question/29809185
#SPJ11
the basic code structure for many sql statements and objects can be found in which section of the sql server management studio?
Answers
The basic code structure for many SQL statements and objects can be found in the "Object Explorer" section of the SQL Server Management Studio. This section allows users to view and manage database objects, including tables, views, stored procedures, and functions, and also provides the ability to generate basic code for these objects.
To view the code for an object in SQL Server Management Studio, you can expand the Object Explorer tree view until you find the object you are interested in. Then, you can right-click on the object and select the "Script [Object] as" option, where [Object] is the type of object you are viewing (e.g., table, view, stored procedure).
Additionally, the "Query Editor" section of the SQL Server Management Studio can be used to create and execute SQL statements, including basic code for creating, modifying, and querying database objects.
Learn more about SQL Statements here:
https://brainly.com/question/29890681
#SPJ11
The basic code structure for many SQL statements and objects can be found in the "Object Explorer" section of the SQL Server Management Studio.
SQL can be used to store information about chemical compounds, including their names, formulas, molecular structures, and physical and chemical properties. This information can be organized into relational databases, which can then be queried using SQL to retrieve specific data sets or to perform complex calculations and analyses.
SQL can also be used to track and manage laboratory data, such as experimental results, analytical data, and instrumentation data. This can facilitate data sharing and collaboration within the scientific community, as well as improve the reproducibility and reliability of experimental results.
To learn more about SQL visit here:
brainly.com/question/13068613
#SPJ4
Which statement is the best description of a chemical reaction? A.The combustion of a substance B.The decomposition of particles C.Mixing two substances D.Forming or breaking bonds
Answers
The answer would be D. Forming or breaking bonds.
When forming or breaking bonds, a new substance is created and it’s no longer just a physical change. Sometimes when bonds are breaking and new ones are forming there can be a change in composition.
Hope this helps !
To solve this we must know the concept of chemical reaction. The correct option is option D that is forming or breaking of bonds. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved.
Chemical reaction is a reaction in which the breaking of old bond from the reactant take place while on the product side, formation of new bond take place to form product.
Therefore, the correct option is option D that is Forming or breaking of bonds take place during chemical reaction.
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ5
Sulfur trioxide gas combined with water to form a single product. SO3 + H2O > ? based on the law of conservation of mass, which option is the product of the reaction?
Answers
Antoine Lavoisier discovered in 1789 that mass is neither created nor destroyed in chemical reactions, which led to the formulation of the Law of Conservation of Mass.
Thus, That is to say, the mass of any one element at the start of a reaction will be equal to that element's mass at the conclusion of the reaction.
The overall mass will remain constant over time in any closed system if we take into account all reactants and products in a chemical reaction. Lavoisier's discovery transformed science and served as the cornerstone for contemporary chemistry.
Because naturally occurring elements are extremely stable under the circumstances present on the Earth's surface, the Law of Conservation of Mass is valid.
Thus, Antoine Lavoisier discovered in 1789 that mass is neither created nor destroyed in chemical reactions, which led to the formulation of the Law of Conservation of Mass.
Learn more about Conservation of mass, refer to the link:
https://brainly.com/question/13383562?
#SPJ1
If 2.22g of NaCl was recovered after the reaction of 0.050L of hydrochloric acid and 0.033L of sodium hydroxide. What was the molarity of the base used in this experiment?
Answers
The molarity of the base used in the experiment, which was determined based on the recovered NaCl and the volumes of hydrochloric acid and sodium hydroxide, was approximately 1.15 M.
To determine the molarity of the base used in the experiment, we need to use the stoichiometry of the balanced chemical equation and the given data.
The balanced chemical equation for the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is:
HCl + NaOH → NaCl + H2O
First, we need to find the number of moles of NaCl produced. We can do this by using the given mass of NaCl (2.22 g) and its molar mass (58.44 g/mol):
moles of NaCl = mass of NaCl / molar mass of NaCl
moles of NaCl = 2.22 g / 58.44 g/mol
moles of NaCl = 0.038 moles
Next, we can use the stoichiometry of the balanced equation to determine the number of moles of NaOH that reacted. Since the mole ratio between NaCl and NaOH is 1:1, the number of moles of NaOH is also 0.038 moles.
Now, we can calculate the molarity of the base (sodium hydroxide) using the given volume of sodium hydroxide solution (0.033 L):
Molarity of NaOH = moles of NaOH / volume of NaOH solution
Molarity of NaOH = 0.038 moles / 0.033 L
Molarity of NaOH ≈ 1.15 M
Therefore, the molarity of the base used in the experiment is approximately 1.15 M.
For more such question on experiment. visit :
https://brainly.com/question/20639065
#SPJ8
consider the tropical ocean is exposed to direct sunlight for many hours everyday if the top 1 cm of area of water 100 km by 100 km warm from 25 to 27 degrees how much heat energy has the water absorbed?
Answers
To calculate the heat energy absorbed by the water, we can use the formula:
Q = mcΔT
Where Q is the heat energy absorbed, m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature.
First, we need to calculate the mass of the water in the top 1 cm of the area:
Volume of top 1 cm of water = 1 cm x 100 km x 100 km = 1 x 10⁸ m³
Density of water = 1000 kg/m³
Mass of water = Volume x Density = 1 x 10⁸ m x 1000 kg/m³ = 1 x 10¹¹ kg
Next, we can calculate the heat energy absorbed using the specific heat capacity of water (4.184 J/g°C):
ΔT = 27°C - 25°C = 2°C
Q = (1 x 10¹¹ kg) x (4.184 J/g°C) x (2°C) = 8.368 x 10¹¹ J
Therefore, the water in the top 1 cm of the 100 km by 100 km area has absorbed approximately 8.368 x 10¹¹ joules of heat energy.
To know more about heat capacity, visit :
https://brainly.com/question/28302909
#SPJ1
Classify each chemical reaction:Reaction2H₂O₂(1)→ 2H₂O(1) + 0₂ (8)K₂SO4 (aq) + Ba(NO3)₂(aq) → 2KNO3(aq) + BaSO4(s)PbCl₂ (aq) + FeSO4 (aq) → FeCl₂ (aq) + PbSO4(s)Typechoose onechoose one✓ choose onecombinationdecompositionsingle substitutionmetathesisnone of the aboveO
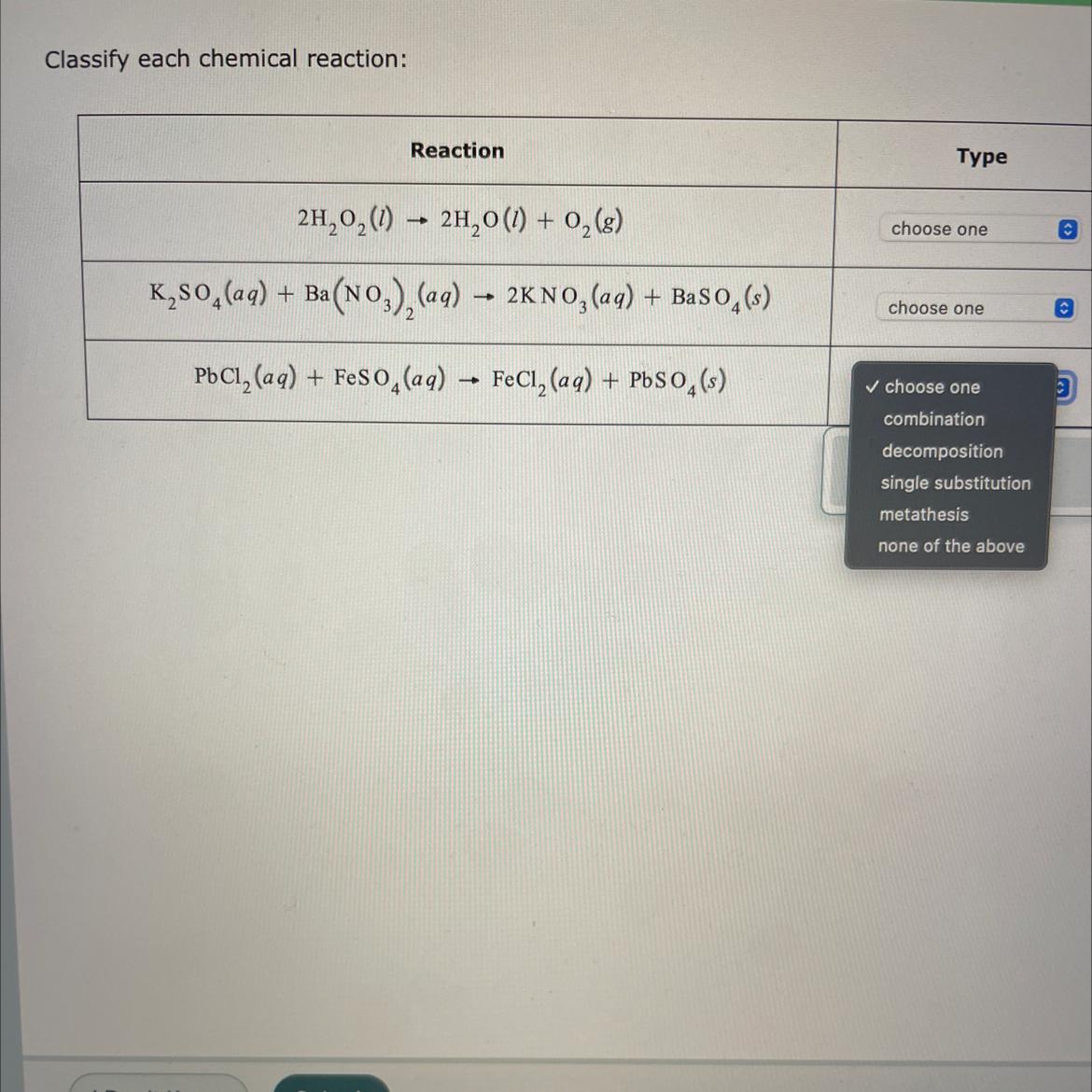
Answers
A decomposition reaction is a chemical reaction in which one reactant breaks down into two or more products.
A metathesis reaction is a chemical reaction in which the positive ions and negative ions present in the reactants appear to exchange partners.
Is it okay if you help me? I'm kind of in a tight spot here. Tysmmmmmmmmmmmmm o(❁´◡`❁)o!!

Answers
Answer:
Explanation:
My guess is option c
Which TWO of the following options are TRUE about atomic mass and mass number?
A - The mass number is always the atomic mass rounded to the nearest whole number.
B - The atomic mass of an element is always the same no matter what the mass number of that particular atom is.
C - Mass number is always equal to the atomic number multiplied by 2.
D - The mass number is written on the periodic table.
E - Atomic mass is calculated by weighted atomic average using all the isotope data available.
F - Atomic mass and mass number are the same thing.
G - Mass number is equal to the sum of protons and electrons in an atom.
PLEASE ANSWER FAST
Answers
Answer:
E - Atomic mass is calculated by weighted atomic average using all the isotope data available.
G - Mass number is equal to the sum of protons and electrons in an atom.
Explanation:
Take an element
\(\\ \bull\sf\longmapsto _{25}Mg^{12}\)
Mass no is 25 and atomic no is 12.1. explain how to change a dilute solution to a concentrated solution 2. what is the concentration of a solution expressed in g/100 ml, of 25 g of solute is dissolved in 40 ml of water 3. why is water referred to as the universal solvent? is this description of water accurate (THIS IS SCIENCE)
Answers
Answer:
1)By adding solute to the solution
2)25g/40ml =5g/8ml
3)Because all soluble solutes dissolve in water