Combustion engines that power vehicles indirectly give rise to which gas that is beneficial in the atmosphere, but also harmful in the troposphere? Select one: a. N2O, nitrous oxide b. CFC, chlorofluorocarbon c. O3, ozone d. CO, carbon dioxide
Answers
The correct answer is d. CO2, carbon dioxide, while carbon dioxide is beneficial in the stratosphere for its role in forming ozone, it is harmful in the troposphere where it contributes to climate change and is distinct from ozone pollution.
Combustion engines, commonly found in vehicles, release carbon dioxide (CO2) as a byproduct of the combustion process. Carbon dioxide is considered a greenhouse gas and contributes to global warming and climate change when it accumulates in the atmosphere. Excessive levels of CO2 trap heat in the Earth's atmosphere, leading to the greenhouse effect.
While carbon dioxide is harmful in the troposphere when it contributes to climate change, it is also beneficial in the upper atmosphere or stratosphere. In the stratosphere, high-energy ultraviolet (UV) radiation from the sun breaks down oxygen molecules (O2) into individual oxygen atoms. These atoms then combine with other O2 molecules to form ozone (O3) through a natural process called the ozone-oxygen cycle. Ozone is crucial in the stratosphere as it absorbs the majority of the sun's harmful UV radiation, protecting life on Earth.
However, in the troposphere, the lower part of the atmosphere where we live, high levels of ozone are harmful. Tropospheric ozone is formed through chemical reactions involving nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the presence of sunlight. It is a harmful air pollutant that can cause respiratory problems, damage crops, and contribute to the formation of smog.
Therefore, while carbon dioxide is beneficial in the stratosphere for its role in forming ozone, it is harmful in the troposphere where it contributes to climate change and is distinct from ozone pollution.
Learn more about stratosphere from below link
https://brainly.com/question/28097222
#SPJ11
Related Questions
2. How many mi hr is 30km/s?
Answers
The answer is 67 108.0888km/s.
what is the citric acid concentration in a soda if it requires 32.27 ml of 0.0148 m naoh to titrate 25.00 ml of soda?
Answers
To determine the concentration of citric acid in a soda, we can use the concept of acid-base titration.
In this case, we are given the volume and concentration of the sodium hydroxide (NaOH) solution used to titrate a known volume of soda. Using this information, we can calculate the concentration of citric acid in the soda. The balanced chemical equation for the reaction between citric acid and sodium hydroxide is: 3 NaOH + H3C6H5O7 → Na3C6H5O7 + 3 H2O. From the balanced equation, we can see that 3 moles of NaOH react with 1 mole of citric acid (H3C6H5O7). Therefore, the number of moles of citric acid in the soda is: moles of citric acid = (moles of NaOH) / 3, moles of NaOH = concentration of NaOH x volume of NaOH used, moles of NaOH = 0.0148 M x 32.27 mL = 0.4757 mmol, moles of citric acid = 0.4757 mmol / 3 = 0.1586 mmol. Next, we can calculate the concentration of citric acid in the soda using the equation: concentration of citric acid = moles of citric acid / volume of soda used, concentration of citric acid = 0.1586 mmol / 25.00 mL = 0.006344 M. Therefore, the concentration of citric acid in the soda is 0.006344 M or 6.344 mM.
To learn more about citric acid click the link below
brainly.com/question/29857075
#SPJ4
When naming acids the prefix hydro- is used when the name of the acid anion ends in___
Answers
Answer:
hydrochloric acid
Explanation:
These anions usually have the ending “-ide.” As acids, these compounds are named starting with the prefix “hydro-,” then adding the first syllable of the anion, then the suffix “-ic.” For example, HCl, which is hydrogen and chlorine, is called hydrochloric acid. More complex acids have oxygen in the compound.
Explain how an ionic compound forms from Aluminum and Sulfur
Answers
An ionic compound is formed when aluminum reacts with sulfur.
When this happens, the aluminum atom loses electrons and becomes positively charged (cations).
The sulfur atom gains electrons and becomes negatively charged (anions).
These ions are then attracted to each other, and this attraction creates the ionic bond.
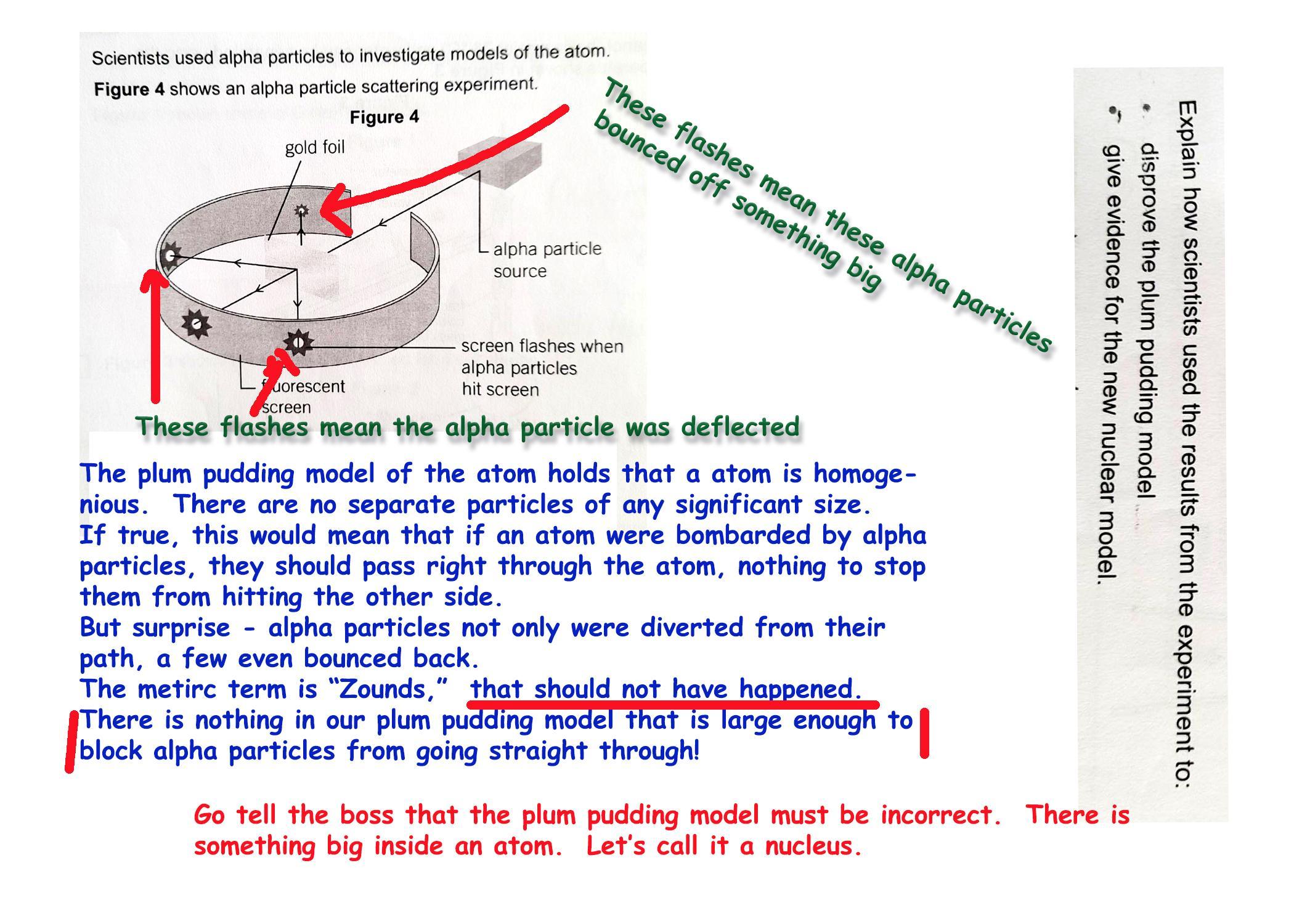
Scientists used alpha particles to investigate models of the atom. Figure 4 shows an alpha particle scattering experiment.
Explain how scientists used the results from the experiment to :
disprove the plum pudding model
give evidence for the new nuclear model

Answers
Answer:
See attached image.
Explanation:
calculate the quantity of heat released in kj when 15.7 g of benzene in the liquid phase at 50.0 °c is converted to solid benzene at 2.0 °c. molar mass of benzene
Answers
The quantity of heat released in kJ when 15.7 g of benzene in the liquid phase at 50.0 °C is converted to solid benzene at 2.0 °C is 3.32 kJ.
What is the quantity of heat released?The quantity of heat released is calculated using the formula below:
The quantity of heat released = (heat evolved from 50 °C to 5.4 °C) + (latent heat of fusion) + (heat evolved on cooling from 5.4 °C to 2.0 °C)The following values apply to benzene:
Melting point = 5.4 °CBoiling point = 90.1 °CHeat of fusion = 9.9 kJ/molHeat of vaporization = 30.7 kJ/molSpecific heat (solid) = 1.51 J/g-"CSpecific heat (liquid) = 1.80 J/g "CSpecific heat (gas) = 1.92 J/g-°CMolar mass = 78.11 g/molMoles of benzene = 15.7/78/11 = 0.20 moles
Heat evolved from 50 °C to 5.4 °C = 15.7 * 1.8 * (50 - 5.4) = 1260.4 J = 1.26 kJ
Latent heat of fusion = 0.2 * 9.9 = 1.98 kJ
Heat evolved on cooling from 5.4 °C to 2.0 °C = 15.7 * 1.51 * (5.4 - 2.0) = 80.6 J = 0.08 kJ
quantity of heat released = 1.26 + 1.98 + 0.08 = 3.32 kJ
Learn more about quantity of heat at: https://brainly.com/question/19666326
#SPJ4
A student needs 3.002 mol of silicon dioxide for an experiment. What mass of silicon dioxide (in grams) should the student obtain
Answers
To calculate the mass of silicon dioxide needed, we need to use its molar mass and the given number of moles.
The molar mass of silicon dioxide (SiO2) can be calculated by adding the atomic masses of silicon (Si) and two oxygen (O) atoms:
Molar mass of Si = 28.09 g/mol
Molar mass of O = 16.00 g/mol
Molar mass of SiO2 = (28.09 g/mol) + 2(16.00 g/mol) = 60.09 g/mol
Now, we can calculate the mass of silicon dioxide needed:
Mass = Number of moles × Molar mass
Mass = 3.002 mol × 60.09 g/mol
Mass ≈ 180.3 g
Therefore, the student should obtain approximately 180.3 grams of silicon dioxide for the experiment.
Learn more about silicon ,visit;
https://brainly.com/question/29769911
#SPJ11
why is the electronic stucture written as 1s 2s 2p 3s 3p 4s. ... ? Shell number 3 can be filled 18 electrons but why isn't 3s 3p 3d written?
Answers
Answer:
Explanation:
The notations for electronic structure of the atom is based upon the application of quantum numbers which specifies the order of filling of atomic orbitals by electrons. There are 4 quantum numbers that include...
Principle QN (n) => specifies location of electron as to major energy level or, ring about the atom of interest.
Orbital QN (l) => specifies the shape of the orbital containing the electron
s => sphere => holds 2 electrons
p => figure eight => holds up to 6 electrons
d => overlapping figure eights => holds up to 10 electrons
f => multiple overlapping figure eights => holds up to 14 electrons
The order of filling follows the Aufbau Principle that electrons enter the atomic orbital with the lowest available energy content. This order of filling from low energy to higher energies follows the Aufbau Diagram.
This order theoretically can go to infinity, but the scientific community only knows of 7 major energy levels; i.e., the 7 rows of the periodic table. See attached Aufbau diagram and follow the arrows in order from low energy to high energy. Use superscripts for number of electrons equal to atomic number of element of interest.
H: 1s¹
O: 1s²2s²2p⁴
Al: 1s²2s²2p⁶3s²3p³
Ag: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d⁹

11.All of the following properties of a diamond are physical except...Select one:a. It does not conduct electricity.b. It produces carbon dioxide when burned in pure oxygen.c. It is transparent like glass.d. It is the hardest material.
Answers
Answer
b. It produces carbon dioxide when burned in pure oxygen.
Explanation
The reaction between diamond and oxygen, producing carbon dioxide is not a physical property of diamond, it is a chemical property because breaking and synthesis of chemical bonds occur.
How does cooler air move in relation to warmer air?
Answers
Cool air moves down while hot air rises
help me plz...........

Answers
Answer:
try use Google search
Explanation:
you need to use it
If the mixture in question 1 is in a 3.0 Liter container at 34 °C, what mass (in grams)of oxygen is present?If oxygen was 0.472 atm
Answers
Answer
The mass (in grams) of oxygen present = 1.79 grams.
Explanation
Given
Volume, V = 3.0 L
Temperature, T = 34 °C = (34 + 273.15 K) = 307.15 K
Pressure, P = 0.472 atm
What to find:
The mass (in grams) of oxygen present.
Step-by-step solution:
Step 1: Calculate the moles of oxygen present.
Using the ideal gas law:
\(PV=nRT\)R is the molar gas constant = 0.0820574 L•atm/mol•K.
\(\begin{gathered} 0.472\text{ }atm\times3.0\text{ }L=n(0.0820574\text{ }L•atm/mol•K\times307.15\text{ }K) \\ \\ n=\frac{0.472\text{ }atm\times3.0\text{ }L}{0.0820574\text{ }L•atm/mol•K\times307.15\text{ }K} \\ \\ n=0.056\text{ }mol \end{gathered}\)The moles of oxygen present is 0.056 mol.
Step 2: Convert 0.056 mol oxygen to mass in grams.
The molar mass of oxygen gas = 31.998 g/mol
Using the mole formula below, the mass of oxygen can be calculated as follows:
\(\begin{gathered} Moles=\frac{Mass}{Molar\text{ }mass} \\ \\ \Rightarrow Mass=Moles\times Molar\text{ }mass \\ \\ Mass=0.056\text{ }mol\times31.998\text{ }g\text{/}mol \\ \\ Mass=1.791888\text{ }g\approx1.79\text{ }grams \end{gathered}\)The mass (in grams) of oxygen present = 1.79 grams.
Sponges reproduce using:
A mitosis
B budding
C sexual reproduction
Answers
Answer:
It is B. Budding
Explanation:
Hope this helped have an amazing day!
Identify the Phase Change (H2O in a soap bubble)
A. Melting
B. Vaporization
C. Sublimation
D. Freezing
E. Condensation
F. Deposition
Answers
What is the total mass of the protons in a`typical ammonia (NH3) molecule? The mass of a proton is 1.673 × 10^-24 g, of a neutron 1.675 × 10^-24 g, and of an electron
9.109 × 10^-28 g.
Answers
The total mass of the protons is 1.338 * 10^-23 g
What is a proton?A proton is the positively charged particle in an atom. It is found in the nucleus along with the neutrons and are collectively called the nucleons.
The hydrogen atom has only one proton while the nitrogen atom has seven protons. Thus makes a total of eight protons in the ammonia molecule.
Hence, the total mass of protons in the ammonia molecule =
8 ( 1.673 × 10^-24 g) = 1.338 * 10^-23 g
Learn more about protons:https://brainly.com/question/1252435
#SPJ1
2. A compound containing iron and sulfur was formed by combining 2.233 g of
iron with 1.926 g of sulfur. What is the empirical formula of the compound?
Answers
A compound containing iron and sulfur was formed by combining 2.233 g of iron with 1.926 g of sulfur. Fe\(_2\)S\(_3\) is the empirical formula of the compound.
While the molecular formula provides the precise number of each unique atom present in a molecule, the empirical formula for a compound provides the simplest ratio for the total amount of different atoms present. It constitutes an empirical formula if it has been simplified. The empirical formula is multiplied by the widely used molecular formula.
number of moles of sulfur = 1.926/ 32=0.06
moles of iron = 2.233/56=0.04
the simplest atomic ratio of the sulfur and iron atoms
S=0.06/0.04=1.5
Fe = 0.04/0.04=1
the whole number atomic ratio
S=1.5×2=3
Fe=1×2=2
empirical formula is Fe\(_2\)S\(_3\).
To know more about empirical formula, here:
https://brainly.com/question/14044066
#SPJ1
I need help on this please
thanks

Answers
Answer:
D. The flesh should return to normal after you press it.
Explanation:
The water content stored in the flesh of the fish, paired with the elasticity are both indicators of freshness, and will cause the flesh to return to normal after its been pressed.
What is the major organic product obtained from the following sequence of reactions? OH OCH3 Loom 2 OCH ОН CH,ONa А CH3OH OH нс 3 4 OCH OCH O a. 1 O b.2 O c.3 O d.4
Answers
The major organic product obtained from the following sequence of reactions is option b. 2 OCH3.
Explanation:
The sequence of reactions is as follows:
1. The first step is the reaction of OH with OCH3 to form Loom 2 OCH.
2. The next step is the reaction of Loom 2 OCH with CH3ONa to form CH3OH and OCH.
3. The final step is the reaction of CH3OH and OCH with OCH to form the final product, 2 OCH3.
Therefore, the major organic product obtained from the sequence of reactions is 2 OCH3, which is option b.
I hope this helps! Let me know if you have any further questions.
Learn more about reaction: https://brainly.com/question/11231920
#SPJ11
Use Lewis theory to determine the formula for the compound that forms between each of the following pairs of elements.
PART A.
Ca and Te
Express your answer as a chemical formula.
PART B.
Ba and Cl
Express your answer as a chemical formula.
Answers
The formula for the compound formed between Ca and Te is CaTe₂.
The formula for the compound formed between Ba and Cl is BaCl₂.
What is Lewis theory?Lewis theory, also known as the Lewis electron dot structure or Lewis structure, is a model used to represent the bonding and electron distribution in molecules and ions. It was given by chemist Gilbert N. Lewis.
According to Lewis theory, atoms in a molecule are represented by their atomic symbols, and valence electrons are represented as dots around the atomic symbols. The valence electrons are the outermost electrons which is involved in chemical bonding.
PART A:
According to Lewis theory, calcium (Ca) has two valence electrons and tellurium (Te) has six valence electrons. To form a stable compound, they need to share or transfer electrons.
Ca (2 valence electrons) + Te (6 valence electrons)
Since calcium needs to lose two electrons to achieve a stable octet and tellurium needs to gain two electrons to achieve a stable octet, they can form an ionic compound with a 2:2 ratio of Ca to Te.
The formula for the compound formed between Ca and Te is CaTe₂.
PART B:
Barium (Ba) has two valence electrons, and chlorine (Cl) has seven valence electrons. Again, according to Lewis theory, they will either share or transfer electrons to achieve stability.
Ba (2 valence electrons) + Cl (7 valence electrons)
Barium can lose two electrons to achieve a stable octet, and chlorine needs to gain one electron to achieve a stable octet. They can form an ionic compound with a 1:2 ratio of Ba to Cl.
The formula for the compound formed between Ba and Cl is BaCl₂.
To know more about Lewis theory, refer here:
https://brainly.com/question/14900884
#SPJ4
In an experiment (first order system), the water in a beaker is heated from temperature of 20
∘
C to the boiling point of 100
∘
C. The time taken for the temperature to reach 100
∘
C is 120 seconds. Derive the transfer function of the boiling process.
Answers
The exponential function is always positive, we can conclude that there is no solution to this equation. This implies that the given data is not consistent with a first-order system.
The transfer function of a system describes the relationship between the input and output signals of the system in the frequency domain. However, the boiling process itself does not have a standard transfer function because it is a complex and dynamic phenomenon influenced by various factors such as temperature, pressure, fluid properties, and heat transfer mechanisms.
To derive the transfer function of the boiling process, we need to understand the dynamics of the system. In this case, we have a first-order system where the water in a beaker is heated from a temperature of 20 °C to the boiling point of 100 °C. The time taken for the temperature to reach 100 °C is given as 120 seconds.
To begin, let's define the input and output variables of the system. The input variable is the heating power or energy applied to the beaker, and the output variable is the temperature of the water.
The transfer function is a mathematical representation of the relationship between the input and output of a system. In this case, the transfer function describes how the temperature of the water changes in response to the heating power.
Let's assume the transfer function is represented as G(s), where s is the complex frequency variable.
To derive the transfer function, we can use the time-domain response data provided. The first-order system response to a step input can be described by the following equation:
y(t) = K(1 - e^(-t/τ))
where y(t) is the output (temperature of the water), K is the steady-state gain, t is time, and τ is the time constant.
Given that the temperature reaches 100 °C after 120 seconds, we can substitute the values into the equation:
100 = K(1 - e^(-120/τ))
Simplifying the equation, we have:
1 - e^(-120/τ) = 100/K
Now, let's consider the initial condition where the water temperature is 20 °C at t = 0. Plugging these values into the equation, we have:
20 = K(1 - e^(-0/τ))
20 = K
Substituting this value of K into the previous equation, we get:
1 - e^(-120/τ) = 100/20
1 - e^(-120/τ) = 5
Now, let's solve for τ. Rearranging the equation, we have:
e^(-120/τ) = 1 - 5
e^(-120/τ) = -4
In summary, based on the provided information, it is not possible to derive the transfer function of the boiling process as a first-order system. Further information or clarification is needed to accurately determine the transfer function.
To know more about first-order system, visit:
https://brainly.com/question/33303904
#SPJ11
FILL IN THE BLANK. nucleic acids determine the types of ____________ synthesized within cells.
Answers
Answer:
Nucleic acids determine the types of protein synthesis synthesized within cells.
What are nucleic acids synthesized by?
Viral nucleic acid synthesis is catalyzed by both viral and host enzymes, the relative contribution of which is determined by the type of virus and the specific molecule. Viruses with RNA genomes, except for the retroviruses, synthesize mRNA and replicate their genomes using virus-encoded RNA-dependent RNA polymerases.
See an example below:
Hope this helps :)
Pls brainliest...

Nucleic acids determine the types of proteins synthesized within cells.
In a cell, nucleic acids such as DNA and RNA play crucial roles in storing and transmitting genetic information. This genetic information serves as instructions for producing proteins, which are essential for numerous cellular processes and functions.
The process of protein synthesis begins with the transcription of DNA into RNA, specifically messenger RNA (mRNA). The mRNA then carries this genetic information from the cell nucleus to the ribosomes in the cytoplasm. At the ribosomes, the mRNA's genetic code is translated into a sequence of amino acids, which are the building blocks of proteins. This process is called translation.
The specific order of amino acids in a protein determines its structure and function. Since the genetic information in nucleic acids dictates the amino acid sequences in proteins, nucleic acids are responsible for determining the types of proteins synthesized within cells.
In summary, nucleic acids are essential for the storage and transmission of genetic information that determines the types of proteins synthesized in cells. These proteins play vital roles in cellular structure, function, and regulation, contributing to the overall health and maintenance of an organism.
Know more about proteins here:
https://brainly.com/question/884935
#SPJ11
What is the mass percent (m/m) of glucose in a solution that contains 30.0 g of water and 5.8 g of glucose?
A) 16.2%
B) 19.3%
C) 14.4%
D) 5.8%
Answers
The mass percent (m/m) of glucose in a solution that contains 30.0 g of water and 5.8 g of glucose is 16.2%.
Thus, the correct option is A.
Mass percent is a term used to describe the concentration of a solution. It is defined as the mass of the solute in grams divided by the total mass of the solution in grams, multiplied by 100 percent.
To find the mass percent of glucose in the given solution, we need to calculate the total mass of the solution. The total mass of the solution is given by the sum of the mass of glucose and water:
30.0 g (water) + 5.8 g (glucose) = 35.8 g (total mass of solution)
The mass percent of glucose in the solution is then calculated as follows:
Mass percent (m/m) = (mass of glucose ÷ total mass of solution) × 100 percent
= (5.8 ÷ 35.8) × 100 percent= 16.2 percent
Therefore, the mass percent (m/m) of glucose in the solution is 16.2%.
For more information about mass percent refers to the link: https://brainly.com/question/9904990
#SPJ11
true or false?
all atoms of the same element contain the same number of neutrons
Answers
Answer: The answer is False
Explanation: They can be the same element yet have different numbers of neutrons i'm pretty sure they are called isotopes
In the gravimetric method, is it important that you dissolve your sample in exactly 10 ml of water? why or why not?.
Answers
When utilizing the gravimetric method, it is crucial to completely dissolve your sample in 10 mL of water. A quantitative technique called gravimetric analysis employs the selective precipitation of the component under study from an aqueous solution.
A group of techniques known as gravimetric analysis are employed in analytical chemistry to quantify an analyte based on its mass. Gravimetric analysis is a quantitative chemical analysis technique that transforms the desired ingredient into a substance (of known composition) that can be extracted from the sample and weighed. This is a crucial point to remember.
Gravimetric water content (g) is therefore defined as the mass of water per mass of dry soil. To calculate it, weigh a sample of wet soil, dry it to remove the water, and then weigh the dried soil (mdry). Dimensions of the sample Water is commonly forgotten despite having a density close to one.
To know more about gravimetry, please refer:
brainly.com/question/18992495
#SPJ4
What is the molecular mass of Br 2?
Answers
Answer:
159.808 g/mol
Answer:
159.80 g/mol
Explanation:
79.90×2=159.80g/mol
How many grams of hydrogen are released by the reaction of 1.00 g of aluminum with excess potassium hydroxide solution assuming 100% yield?
Answers
When 1.00 g of aluminum reacts with excess potassium hydroxide solution, how many grams of hydrogen are released assuming a 100% yield? What is aluminum? Aluminum is a chemical element with the symbol Al and atomic number 13. It is a silvery-white, soft, non-magnetic, and ductile metal in the boron group.
Its atomic mass is 26.98, and it is the third-most abundant element, after oxygen and silicon. Potassium hydroxide is an inorganic compound with the formula KOH, commonly known as caustic potash. It is a strong base, and its chemical properties are similar to those of sodium hydroxide, the more well-known caustic soda.1.00 g of aluminum reacts with potassium hydroxide solution as follows:2Al + 2KOH + 6H2O → 2KAl(OH)4 + 3H2From the balanced chemical reaction above, two moles of aluminum react with two moles of potassium hydroxide and six moles of water to produce two moles of potassium tetrahydroxy aluminate and three moles of hydrogen. Therefore, one mole of aluminum reacts with one mole of potassium hydroxide and three moles of water to produce one mole of potassium tetrahydroxy aluminate and 1.5 moles of hydrogen. Using the periodic table, we can determine the molar mass of aluminum to be 26.98 g/mol. Therefore, one mole of aluminum weighs 26.98 g. Using the balanced equation, we can see that one mole of aluminum produces 1.5 moles of hydrogen. Since one mole of hydrogen weighs 1.008 g, we can determine the mass of hydrogen released by 1.00 g of aluminum by using the following formula: Mass of hydrogen = Number of moles of hydrogen × Molar mass of hydrogen Mass of hydrogen = 1.5 × 1.008Mass of hydrogen = 1.51 g Therefore, 1.51 g of hydrogen are released by the reaction of 1.00 g of aluminum with excess potassium hydroxide solution assuming 100% yield. Answer: 1.51 grams
learn more about hydroxide here.
https://brainly.com/question/31820869
#SPJ11
Can anyone help me with this? It's chemistry

Answers
Answer:
ohh... it's time vs air that's all
A 0.425g sample of propane was mixed with excess oxygen in a calorimeter containing 98.72 g of water. The system was ignited and the temperature rose from 18.29ºC to 26.85ºC. The chemical equation for this reaction is :
C3H8 + 02 --> 3CO2 + 4H2O
Calculate the molar enthalpy of propane in J/mol
Answers
A 0.425g sample of propane was mixed with excess oxygen in a calorimeter containing 98.72 g of water. The system was ignited and the temperature rose from 18.29ºC to 26.85ºC. the molar enthalpy of propane is 393851.1 J/mol
The mass of propane = 0.425 g
molar mass of propane = 44 g/mol
moles = mass / molar mass
= 0.425 / 44
= 0.009 mol
ΔH = mc ΔT
ΔH = n ΔHsol
ΔHsol = mc ΔT / n
= 98.72 × 4.18 ( 26.85 - 18.29 ) / 0.009
molar enthalpy of solution = 393851.1 J/mol
Thus, A 0.425g sample of propane was mixed with excess oxygen in a calorimeter containing 98.72 g of water. The system was ignited and the temperature rose from 18.29ºC to 26.85ºC. the molar enthalpy of propane is 393851.1 J/mol
To learn more about molar enthalpy here
https://brainly.com/question/3207013
#SPJ1
I need help ASAP pls

Answers
Which of the following explains why metals are able to be pounded, extruded, bent, and shaped without breaking?
Which of the following explains why nonmetals are typically harder and more brittle than metals?
Answers
Answer: Malleability
Explanation: Is because metals have mobile electrons in their s orbitals.
Hope this helps..
Answer:
The answer to this question is mobile electrons in s orbitals.
The answer to the following question on edg is directional electrons in p orbitals.
Explanation: