coal and petroleum deposits in the earth are limited explain
Answers
Answer:
they're a non renewable source
Explanation:
Coal and petroleum, are formed by the deposit of organisms that have died a million years ago. under pressure and high temperature underground the dead organisms that used to live millions of years ago are formed into petroleum. however, coal is formed by the deposit of dead plants in the same process as petroleum. for the formation of petroleum and coal it takes a long term of time for it to be formed (millions of years). and that's why its limited
Answer:
Hi!!
Coal and petroleum deposits in the earth are limited.
They are non renewable resources. They are formed after a long period. They are formed by the remains of dead plants and animals present deep inside the earth crust. It took millions of years to get converted into coal and petroleum.
Hope it helps!!!!
Related Questions
I would like to get some help on this

Answers
Compound Type of IMFs Sublime at STP?
Carbon dioxide London dispersion Yes
Hydrogen fluoride Hydrogen bonding No
Calcium chloride Ionic bonding No
Naphthalene London dispersion Yes
Iodine London dispersion Yes
Sodium chloride Ionic bonding No
Water Hydrogen bonding No
What is a Compound?A chemical compound is described as a chemical substance composed of many identical molecules containing atoms from more than one chemical element held together by chemical bonds.
London dispersion forces are also described as a type of intermolecular force acting between atoms and molecules that are normally electrically symmetric.
Learn mo0re about intermolecular force at: https://brainly.com/question/2193457
#SPJ1
Restate the 4 laws that govern energy.
Answers
Answer:
here is your answr
Explanation:
The first law of thermodynamics, also known as Law of Conservation of Energy, states that energy can neither be created nor destroyed; energy can only be transferred or changed from one form to another. For example, turning on a light would seem to produceenergy; however, it is electrical energy that is converted.
Answer:
the law of thermodynamics energy cannot be destroyed or created. energy can only be changed or transferred from one form to another. for example turning on a light would be seem produced energy, however electrical energy can be produced
FeBr3+Ba(OH)2——-Fe(OH)3+BaBr2
Answers
Answer:
Balanced Chemical Equation
2FeBr3 + 3Ba(OH)2 → 2Fe(OH)3 + 3BaBr2
Explanation:
Reaction Information
Ferric Bromide + Barium Hydroxide = Iron(III) Hydroxide + Barium Bromide
Reaction Type
Double Displacement (Metathesis)
Reactants
Ferric Bromide - FeBr3
FeBr3
Molar Mass of Br3Fe Oxidation State of Br3Fe
Barium Hydroxide - Ba(OH)2
Caustic Baryta Barium Hydroxide Lime Ba(OH)2 Barium Dihydroxide Hydrate
Molar Mass of BaH2O2 Oxidation State of BaH2O2
Products
Iron(III) Hydroxide - Fe(OH)3
Ferric Hydroxide Ferric Oxide Yellow
Molar Mass of FeH3O3 Oxidation State of FeH3O3
Barium Bromide - BaBr2
Molar Mass of BaBr2 Oxidation State of BaBr2
FeBr3 + Ba(OH)2 = Fe(OH)3 + BaBr2
Instructions
To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above.
Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F.Ionic charges are not yet supported and will be ignored.Replace immutable groups in compounds to avoid ambiguity. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will.Compound states [like (s) (aq) or (g)] are not required.You can use parenthesis () or brackets []i hope it's help you
How do catalysts increase reaction rates? *
By increasing the activation energy.
By broadening the energy barrier.
By forming an activated complex with lower energy.
By changing the net thermodynamics of the reaction.
Answers
Answer:
By forming an activated complex with lower energy.
Explanation:
When catalyst is added to a reaction , it forms an activated catalyst which has lower activation energy . So initiation of reaction requires less energy and reaction becomes fast .
Hence third option is correct.
Can someone help me with my chemistry problem?

Answers
Answer:
D. 108g of water
Explanation:
16g CH4 produces 2(18)g of H20
1g CH4 produces \(\frac{36}{16}\)g of H2O
48g CH4 produces \(\frac{36}{16}\)×48
108g of H20
Answer:
D. 108g of water
Explanation:
why should you repeat the experiment of preparing soluble salts by titration without using an indicator before boiling it?
Answers
Answer:
Explanation:
Titration: titrate twice, the first time with an indicator to determine how much sodium hydroxide is needed to completely react with hydrochloric acid, and the second time without an indicator to prevent the contamination of the sodium chloride salt produced
The molar solubility of lead(II) fluoride (PbF2) is 2.1 x 10-3 mol/L in pure water at 25oC. What is the molar solubility of lead(II) fluoride in 0.050 M NaF at 25oC
Answers
The molar solubility of lead(II) fluoride in 0.050 M NaF at 25°C is approximately 1.4 x 10⁻⁵ mol/L.
The solubility of a compound can be affected by the presence of other ions in solution, which can cause the equilibrium to shift. In this case, the presence of NaF will provide additional fluoride ions (F⁻) in the solution, which can potentially affect the solubility of lead(II) fluoride (PbF₂).
To determine the molar solubility of PbF₂ in 0.050 M NaF, we need to consider the common ion effect. The additional fluoride ions from NaF can react with Pb²⁺ ions to form PbF₄²⁻ complex ions, reducing the concentration of Pb²⁺ ions in solution and affecting the solubility equilibrium.
Since the molar solubility of PbF₂ in pure water is given as 2.1 x 10⁻³ mol/L, we can assume that the concentration of Pb²⁺ ions is also 2.1 x 10⁻³ mol/L in the presence of excess F⁻ ions.
Using the balanced equation for the dissolution of PbF₂: PbF₂(s) ⇌ Pb²⁺(aq) + 2F⁻(aq), we can establish the solubility product expression: Ksp = [Pb²⁺][F⁻]².
Substituting the known values, we have: Ksp = (2.1 x 10⁻³)(2.1 x 10⁻³)².
Now, considering the presence of 0.050 M NaF, the concentration of F⁻ ions in solution will be 0.050 M. Since the concentration of Pb²⁺ ions will be reduced due to the formation of PbF₂⁻ complex ions, we can assume it to be x M.
Using the solubility product expression again, we have: Ksp = (x)(0.050)².
Equating the two expressions for Ksp, we can solve for x, which represents the molar solubility of PbF₂ in 0.050 M NaF.
(x)(0.050)² = (2.1 x 10⁻³)(2.1 x 10⁻³)².
Solving the equation, the molar solubility of PbF₂ in 0.050 M NaF is approximately 1.4 x 10⁻⁵ mol/L.
To learn more about molar solubility, here
https://brainly.com/question/31043999
#SPJ4
If one mole of atoms or molecules is equal to 6.022 × 1023 atoms or molecules, how many molecules are in a 23.45 g sample of copper (ii) hydroxide, cu(oh)2? (mm of cu(oh)2 is 97.562 g/mol)
Answers
The number of molecules that are needed in a 23.45 g sample of copper (ii) hydroxide, are 1.45 x 10²³.
What is Avogadro's number?Avogadro's number is the measurement of the one mole of any substance that is equal to the Avogadro's number.
Avogadro's number is 6.022 × 10²³.
To calculate the moles
Moles = mass / molar mass
Mass is 23.45 g
Molar mass is 97.562
Putting the value in equation
Moles = 23.45 g/ 97.562 = 0.24 mol.
The moles of copper (II) hydroxide has been 0.24 mol.
The number of molecules in 0.24 mol sample has been driven by:
1 mol = 6.022 × 10²³.
0.24 mol = 0.24 x 10²³.
0.24 mol = 1.45 x 10²³.
Thus, the number of molecules that are needed in a 23.45 g sample of copper (ii) hydroxide, are 1.45 x 10²³.
Learn more about Avogadro's number
https://brainly.com/question/1445383
#SPJ1
Which of the following are products when magnesium metal is placed in hydrochloric acid? (Correct answer should be D but why)
A-H
B-H+
C-Mg
D-MgCl2
Answers
Answer:
an acid +metal =salt +hydrogen
Explanation:
HCL+Mg =Mgcl2+H2
(because Mg has an ion with a +2 charge ,it attracts Cl with a -2 charge )
therefore the correct answer is D for the above reasons
Answer:
When magnesium reacts with hydrochloric acid (HCl), a single-replacement reaction occurs. These reactions involve the substitution of one element in a compound with another. In this case, the hydrogen in HCl will be swapped with the magnesium metal because both of these elements make cations (positively-charged ions) when they participate in ionic bonding.
So why does the chorine have a subscript of 2 when it bonds with magnesium? This occurs in order to balance the ionic charges and make the overall compound neutral.
Magnesium wants to give away 2 electrons when it ionizes, forming the cation Mg²⁺. However, chlorine only wants to gain 1 electron to fill its valence shell, making it form the anion, Cl⁻. As you can see, if just one of each ion were to bond, the compound would have an overall charge of +1 because (+2 - 1 = +1). Therefore, the compound can be made neutral if two chlorine ions bond with just 1 magnesium ion (+2 - 1 - 1 = 0).
The hydrogen ion from HCl becomes H₂ after the reaction occurs. This occurs because hydrogen generally exists as a diatomic compound in nature (diatomic = exists as 2 atoms).
The complete balanced equation for the reaction is:
Mg + 2 HCl ------> MgCl₂ + H₂
How many oxygen atoms are in 15 molecules of tetraphosphorus decoxide (also known as phosphorus pentoxide), p₄o₁₀?
Answers
There are 150 oxygen atoms in 15 molecules of tetraphosphorus decoxide (P₄O₁₀).
Tetraphosphorus decoxide (P₄O₁₀) consists of four phosphorus atoms and ten oxygen atoms. To determine the number of oxygen atoms in 15 molecules of P₄O₁₀, we can first calculate the total number of oxygen atoms in one molecule and then multiply by 15.
The subscript "10" in P₄O₁₀ indicates that there are ten oxygen atoms. Therefore, in one molecule of P₄O₁₀, we have:
10 oxygen atoms
To find the number of oxygen atoms in 15 molecules of P₄O₁₀, we multiply the number of oxygen atoms per molecule by the number of molecules:
Number of oxygen atoms = 10 oxygen atoms/molecule × 15 molecules
Number of oxygen atoms = 150 oxygen atoms
So, there are 150 oxygen atoms in 15 molecules of tetraphosphorus decoxide (P₄O₁₀).
learn more about tetraphosphorus here
https://brainly.com/question/33450831
#SPJ11
Propane burns in excess oxygen according to the following reaction. C3H8 +502 - 3CO2 +4H2O
a. How many moles each of CO2 and H2O are formed from 3. 85 mol of propane?
Answers
From 3.85 moles of propane, 11.55 moles of carbon dioxide and 15.40 moles of water are formed.
From the balanced equation, we can see that for every 1 mole of propane (C3H8) that reacts, 3 moles of carbon dioxide (CO2) and 4 moles of water (H2O) are formed.
Given that we have 3.85 moles of propane, we can calculate the moles of carbon dioxide and water produced using the mole ratios:
Moles of CO2 = 3.85 mol propane × (3 mol CO2 / 1 mol propane) = 11.55 mol CO2
Moles of H2O = 3.85 mol propane × (4 mol H2O / 1 mol propane) = 15.40 mol H2O
Therefore, from 3.85 moles of propane, 11.55 moles of carbon dioxide and 15.40 moles of water are formed.
learn more about propane here
https://brainly.com/question/14519324
#SPJ11
A solution containing 572. 0ml of 0. 6300mhcl is diluted to a volume of 1. 000l. what is the ph of this solution?
Answers
The pH of the solution is approximately 0.444.
To find the pH of the solution, we need to first determine the concentration of the diluted solution.
Given:
Initial volume (V1) = 572.0 mL
Initial concentration (C1) = 0.6300 M
Final volume (V2) = 1.000 L
We can use the dilution formula to find the concentration of the diluted solution:
C2 = (C1 * V1) / V2
Substituting the given values:
C2 = (0.6300 M * 572.0 mL) / 1.000 L
C2 = 0.3604 M
Now, we can use the formula for calculating pH, which is given by:
pH = -log[H+]
Since HCl is a strong acid, it completely dissociates into H+ ions. Thus, the concentration of H+ ions in the solution is equal to the concentration of the HCl.
Therefore, the pH of the solution is:
pH = -log(0.3604)
pH ≈ 0.444
So, the pH of the solution is approximately 0.444.
Learn more about strong acid here:
https://brainly.com/question/29769012
#SPJ11
Which has a greater atomic radius silicon or magnesium
Answers
3. Briefly discuss the results of the TLC. Was there evidence of unreacted p-nitrobenzaldehyde in either product
Answers
TLC means Thin Layer Chromatography. It is a method that can best be described as "Affinity-Based" used in the separation of compounds that are in a mixture.
What is unreacted p-nitrobenzaldehyde?Unreacted p-nitrobenzaldehyde is simply an organic aromatic compound that contains a nitro group para-substituted to an aldehyde. in this case, if it is unreacted, that means it is the same as before the chemical reation.
Note that the question is missing key information hence the general answer.
Learn more about TCL at:
https://brainly.com/question/10296715
Someone pls help me I will make you brain

Answers
Answer:3
Explanation:
can you pair electrons in degenerate orbitals before filling each orbital halfway
Answers
5. Which coefficients correctly balance the formula equation
Zn(OH)2 + CH3COOH → Zn(CH3COO)2 + H2O?
a. 1, 2, 1, 1
b. 2, 1, 2, 1
b. 1, 2, 1, 2
d. 2, 1, 1, 2
Answers
How is a rainbow made
Answers
Please help I will mark brainy
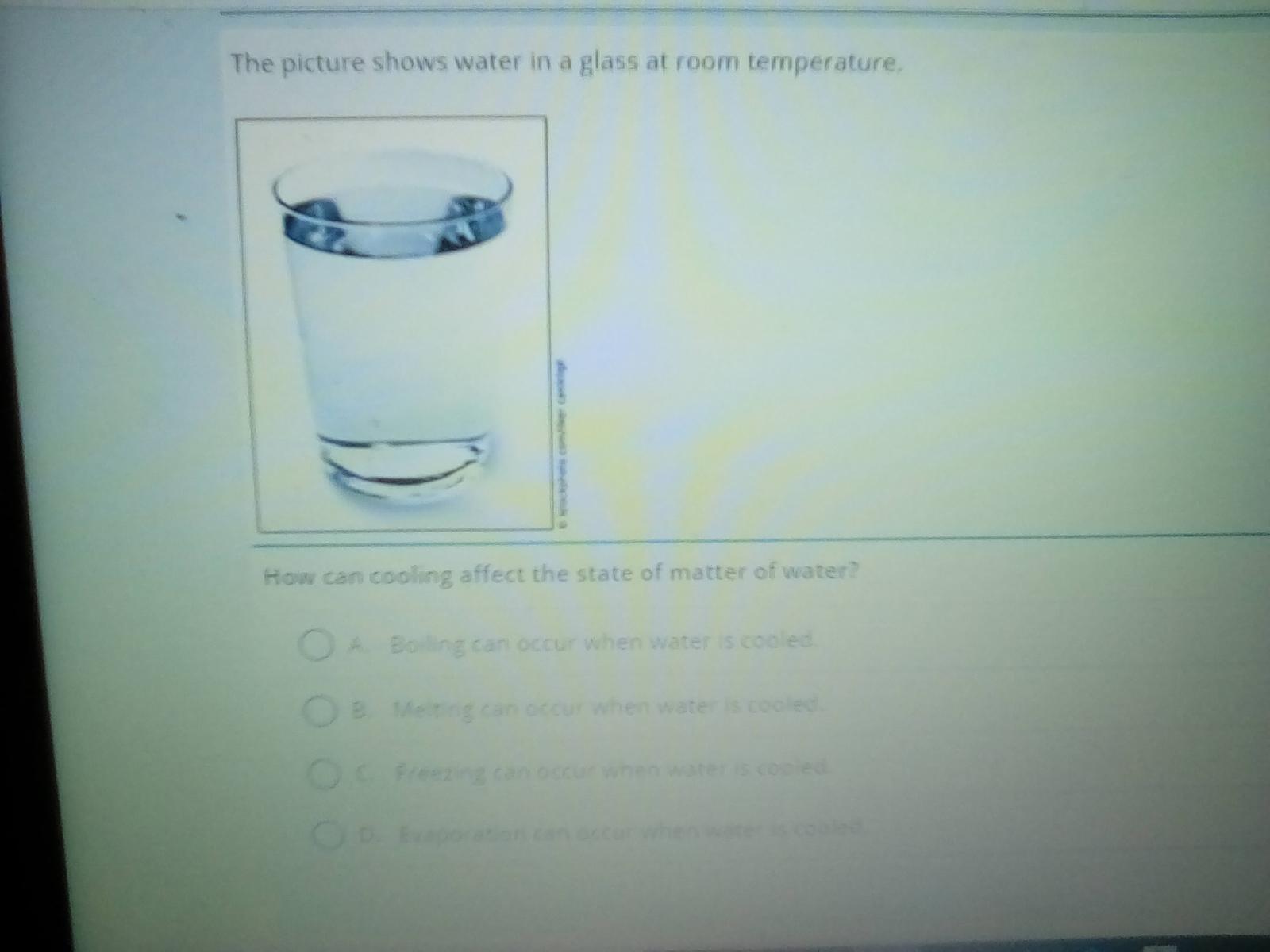
Answers
Answer:freezing can occur
Source:trust me bro
How much energy is required to raise 10.0 grams of water (c=4.18j/gC) by 20*C?
Answers
Answer:
Q = 836 J
Explanation:
Given data:
Mass of water = 10.0 g
Temperature increased =ΔT = 20°C
Specific heat capacity of water = 4.18 J/g.°C
Solution:
Formula:
Q = m.c. ΔT
Q = amount of heat absorbed or released
m = mass of given substance
c = specific heat capacity of substance
ΔT = change in temperature
Q = 10.0 g × 4.18 J/g.°C × 20°C
Q = 836 J
At STP, 5.6 liters of CH4 contains the same number of molecules as
1.4 L of oxygen
2.8 L of ammonia
5.6 L of hydrogen
11.2 L of neon
Answers
5.6 L of \(CH_{4}\) contains the same number of molecules 5.6 L of \(H_{2}\).
Define mole.
The mole is always the SI unit used to measure a substance's quantity.
Define molecules.
The smallest particle of a substance. It contains 2 or more atoms.
5.6 L of \(CH_{4}\):
No of moles=Given volume/molar volume
= \(5.6/22.4\)
= \(0.25\)
No of molecules=No of moles*Avagadro's number
= \(0.25*6.023*10^{23}\)
= \(1.51*10^{23}\)
5.6 L of \(H_{2}\):
No of moles=Given volume/molar volume
= \(5.6/22.4\)
= \(0.25\)
No of molecules = No of moles * Avagadro's number
= \(0.25*6.023*10^{23}\)
= \(1.51*10^{23}\)
Therefore, the answer is 5.6 L of \(H_{2}\).
To learn more about Moles and molecules, the given link https://brainly.com/question/21911991.
#SPJ1
1. The equilibrium constant, Kc, for the following reaction is 1.89×10-6 at 506 K.
NH4Cl(s) NH3(g) + HCl(g)
Calculate Kc at this temperature for the following reaction:
NH3(g) + HCl(g) NH4Cl(s)
Kc =
Answers
If the equilibrium constant, Kc, for the following reaction is 1.89 × 10⁻⁶at 506 K, Kc for the reaction: NH3(g) + HCl(g) → NH4Cl(s)is 5.291 × 10⁵.
The given reaction is: NH4Cl(s) → NH3(g) + HCl(g)
To calculate Kc for the reaction: NH3(g) + HCl(g) → NH4Cl(s) we must reverse the given reaction. We must use the relationship: If aA + bB ⇌ cC + dD then Kc = [C]c[D]d/[A]a[B]b where [A], [B], [C], [D] are molar concentrations at equilibrium, while a, b, c, d are the coefficients of the balanced chemical equation. To reverse the given reaction, we must invert the value of the Kc. The equation is: NH4Cl(s) ⇌ NH3(g) + HCl(g)Kc = 1.89 × 10⁻⁶at 506 K
Now, we will invert this equation. Kc(NH3)(HCl) = 1/Kc(NH4Cl)
Now, substituting the values: Kc(NH3)(HCl) = 1/(1.89 × 10⁻⁶)Kc(NH3)(HCl) = 5.291 × 10⁵
Therefore, Kc for the reaction:NH3(g) + HCl(g) → NH4Cl(s)is 5.291 × 10⁵.
More on equilibrium constant: https://brainly.com/question/30620209
#SPJ11
what is the angle of the tetrahedral bond?
Answers
109.5
when we see it in 3d dimension...the repulsion force of the bonding pairs maximaze the distance from each other, thus making the angle 109.5
The frequency of a wave is 2.8 x 10^3 Hertz. What is the wavelength of these gamma rays?
Show all work!
Answers
The frequency of a wave 2.8 x 10³ Hertz is then the wavelength of these gamma rays is 107 nm
Wavelength is the the distance between two successive crests or troughs of a wave
Here given data is
Frequency = 2.8 x 10³ Hertz
Velocity of gamma rays = 3×10⁸m/s
We have to calculate wavelength of these gamma rays?
So, wavelength = velocity/frequency
λ = v/f
λ = 3×10⁸m/s/2.8 x 10³ Hertz
λ = 107 nm
Know more about wavelength
https://brainly.com/question/11483057
#SPJ1
what is the term for the component of a solution that is the greater quantity?
Answers
The term for the component of a solution that is present in the greater quantity is the solvent, as in a solution, the solvent refers to the component that is present in a larger quantity or amount compared to the other component, which is called the solute.
The solvent is the substance that dissolves the solute to form a homogeneous mixture. It is typically a liquid, but it can also be a gas or a solid. The solute, on the other hand, is the substance that is dissolved within the solvent. The solvent provides the medium in which the solute particles are dispersed and dissolved. It determines the physical state (such as liquid or gas) of the solution. The solute, being present in a lesser quantity, becomes evenly distributed within the solvent particles.
Learn more about the solution here.
https://brainly.com/question/1616939
#SPJ1
How many formula units are in 1.58 moles of LiOH ?
A)
2.53E24
B)
3.79E24
0)
3.49E25
D)
9.51E23
Answers
There are D) 9.51E23
Further explanationGiven
1.58 moles of LiOH
Required
The number of formula units
Solution
The mole is the number of particles(molecules, atoms, ions, formula units) contained in a substance
1 mol = 6.02.10²³ particles
Can be formulated
N=n x No
N = number of particles
n = mol
No = Avogadro's = 6.02.10²³
So for 1.58 moles :
= 1.58 x 6.02 x 10²³
= 9.5116 x 10²³
The
of bomb calorimeter is constant.
temperature
pressure
volume
Answers
Answer: Your question doesen't make since and you should word it better but what I think you are asking is...
The ___ of a bomb calorimete is constant, what is the temperatue, pressure and volume of it?
Explanation: GL to the next person who tries to answer this.
what element is a F-18 Atom
Answers
Answer:
Sodium Fluoride F 18 Injection is a positron emitting radiopharmaceutical, no-carrier added.
Explanation:
Element Name Fluorine
Element Symbol F
Atomic Number 9

Matter and your body-how are they
interrelated?
Answers
Answer:
Matter has mass and takes up space, everything has matte, your body takes up space. Matter refuels the body. You can also so your body produces matter as well
Why are lanthanides and actinides placed in separate rows at the bottom of the periodic table
Answers
Answer:
The reason why the YMCA is a good one I can get it to me if t