Choose the metal that is produced by electrolysis of its molten chloride salt. select one: a. mg b. ca c. sr d. ba e. all of these
Answers
The correct answer is [b] calcium is the metal that is produced by electrolysis of its molten chloride salt.
What is a metal?Metals are the opaque, lustrous elements that are good conductors of heat and electricity. Most metals are malleable and ductile and are, in general, denser than other elemental substances
Any of various opaque, fusible, ductile, and typically lustrous substances that are the good conductors of electricity and heat, form cations by loss of electrons, and yield basic oxides and hydroxides especially :the one that is a chemical element as distinguished from an alloy.
A metal may be a chemical element such as iron; an alloy such as stainless steel; or molecular compound such as polymeric sulfur nitride.
In physics, a metal is generally regarded as any substance capable of conducting the electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under the high pressures.
To know more about metals visit: brainly.com/question/18153051
#SPJ4
Related Questions
Consider this reaction: Upper K Upper O Upper H + Upper H Upper B 4 right arrow Upper K Upper B r + Upper H Subscript 2 Baseline Upper O
Which is the acid in this reaction?
KOH
HBr
KBr
H2O
Answers
Answer:
HBr
Explanation:
What is the binding energy b of the last neutron of silicon‑30? the atomic mass of silicon‑30 is 29. 973770 u, whereas the atomic mass of silicon‑29 is 28. 976495 u
Answers
The binding energy of the last neutron in silicon-30 is 2.346 × 10^-12 J.
The binding energy of a nucleus is the energy required to separate all of its constituent nucleons (protons and neutrons) from each other to an infinite distance. The binding energy per nucleon is a measure of the stability of a nucleus, with higher values indicating greater stability.
To calculate the binding energy of the last neutron in silicon-30, we need to use the atomic masses of silicon-30 and silicon-29 to determine the mass defect of silicon-30:
mass defect = (atomic mass of protons and neutrons) - (atomic mass of nucleus)
The atomic mass of silicon-30 is 29.973770 u, and the atomic mass of silicon-29 is 28.976495 u. Therefore, the mass defect of silicon-30 is:
mass defect = (30 protons + 30 neutrons) × 1.008665 u - 29.973770 u
mass defect = 0.259625 u
This means that the total binding energy of the silicon-30 nucleus is:
binding energy = mass defect × c^2
where c is the speed of light in a vacuum, which is approximately 2.998 × 10^8 m/s.
binding energy = 0.259625 u × (1.66054 × 10^-27 kg/u) × (2.998 × 10^8 m/s)^2
binding energy = 2.335 × 10^-11 J
Since we are interested in the binding energy of the last neutron in silicon-30, we need to subtract the binding energy of the silicon-29 nucleus (which has 29 neutrons) from the binding energy of the silicon-30 nucleus:
binding energy of last neutron = binding energy of silicon-30 nucleus - binding energy of silicon-29 nucleus
binding energy of last neutron = (30 nucleons × 2.335 × 10^-11 J) - (29 nucleons × 2.308 × 10^-11 J)
binding energy of last neutron = 2.346 × 10^-12 J.
Learn more about binding energy
https://brainly.com/question/30073915
#SPJ4
Explain how copper is produced from copper() sulfate solution by electrolysis?
Answers
Answer: Copper is purified by electrolysis . Electricity is passed through solutions containing copper compounds, such as copper(II) sulfate. The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. Pure copper forms on the cathode.
you consider starting material a. you know that a can undergo two irreversible reactions as shown in the below reaction coordinate diagram with one reaction pathway labeled in red and one reaction pathway labeled in blue. the red path leads to product b, while the blue path leads to product c. assuming both reaction pathways occur simultaneously in competition with each other, what is the major product, and why?
Answers
Product B because it has a lower energy level than Product C's transition state, which leads to Product C.
What are reaction pathways?The series of reactions required to create a desired product are described by a reaction pathway. The distribution strategy for a product is determined by things like percentage yield. Atomic economics. reaction time. is a connected graph with chemical species as its nodes. If a reaction transfers material from one species to the other, an edge unites the two. An vector from reactant toward the product is depicted as the edge.
What role do reactions pathway ?Energy, or ATP, is created by chemical reactions within our cells. All living things require energy to survive, and Adp would be a reactant that fuels a number of other chemical reactions inside cells. Cells generate energy through a process called cellular respiration.
To know more about Reaction pathways visit:
https://brainly.com/question/16047003
#SPJ4
Which atomic model came first? A.Daltons BB.Bohrs C.Thomson’s D.Rutherfords
Answers
Answer:John Dalton
Explanation:
Why does Xenon not react with Nitrogen, (in simple terms)
Answers
Explanation:
because Xenon is Noble gas
3. The program can potentially
even send drones to spray a
substance that can slow the
spread of fire?
Answers
Answer:
h2-+2945-5456vjemrnfn
What is the number of significant figures in each of the following measured quantities? 0.0105 L.
Answers
The measured quantity 0.0105 L has three significant figures. Significant figures are the digits in a measurement that convey precision, excluding leading zeros and trailing zeros without a decimal point.
In the measured quantity 0.0105 L, there are three significant figures. Significant figures are the digits in a measurement that indicate the precision and reliability of the value. The general rule for determining significant figures is as follows:
1. Non-zero digits are always significant. In this case, the digits "1", "0", and "5" are all non-zero and therefore significant.
2. Leading zeros (zeros at the beginning of a number) are not significant; they act as placeholders. In this measurement, the leading zero before the decimal point is not considered significant.
3. Zeros between significant digits are significant. There are no zeros between the significant digits "1", "0", and "5" in this case.
4. Trailing zeros (zeros at the end of a number) after a decimal point are significant. In this measurement, the trailing zero after the "5" is significant.
By applying these rules, we can determine that the measured quantity of 0.0105 L has three significant figures, representing the precision of the measurement to the hundredth place.
learn more about measured quantity here:
https://brainly.com/question/29135463
#SPJ11
The photograph shows salt. Where does salt come from?
O A. The melting of metamorphic rock
B. The weathering of sediment
O C. The crystallization of magma
O D. The precipitation of sedimentary rock
Answers
Answer:
c. The crystallization of magma
Hope that helps! :)
Explanation:
6. In which of the following will the bulb glow?
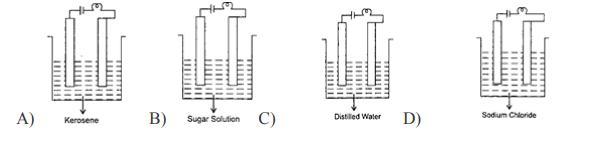
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later
KI E) AIBrs (181 Major 2, Q17) If you have two molecule of TiO2, how many molecules of O2 does it contain? A) Zero. B) One. C) Two. D) Three E) Four. (181 Major 2, Q21) Prepared by Abul Lais
Answers
To determine the number of molecules of oxygen in two molecules of titanium dioxide, we need to consider the composition of . each molecule of oxygen consists of two oxygen atoms, the number of molecules of oxygen would be: 2 molecules of O2 Correct answer is option C
The chemical formula for titanium dioxide indicates that each molecule of titanium dioxide consists of one titanium (Ti) atom and two oxygen (O) atoms. This means that each molecule of titanium dioxide contains two oxygen atoms.
Given that we have two molecules of titanium dioxide, we can calculate the total number of oxygen atoms by multiplying the number of molecules by the number of oxygen atoms per molecule: 2 molecules of titanium dioxide* 2 oxygen atoms/molecule = 4 oxygen atoms Therefore, two molecules of titanium dioxide contain a total of four oxygen atoms.
Since each molecule of oxygen consists of two oxygen atoms, the number of molecules of O2 would be:4 oxygen atoms / 2 oxygen atoms/molecule = 2 molecules of oxygen So, the correct answer is option C)
Know more about titanium dioxide here:
https://brainly.com/question/27743149
#SPJ11
if you have one molecule of tio2, how many molecules of o2 does it contain?
Answers
A single molecule of TiO2 (titanium dioxide) contains one titanium (Ti) atom and two oxygen (O2) atoms. However, it does not contain any O2 molecules, as O2 molecules consist of two oxygen atoms bonded together. In TiO2, the oxygen atoms are bonded to the titanium atom instead.
Titanium dioxide (TiO2) is a compound consisting of one titanium atom (Ti) and two oxygen atoms (O). Therefore, if you have one molecule of TiO2, it contains two molecules of O2.In TiO2, the titanium atom is bonded to two oxygen atoms through covalent bonds. Each oxygen atom contributes two electrons to form the covalent bonds with the titanium atom. This results in a stable structure with two oxygen atoms for each molecule of TiO2.
To break down the molecular composition further, the two oxygen atoms in TiO2 are not free O2 molecules. Instead, they are part of the chemical structure of TiO2 and are bonded to the titanium atom. The representation "O2" refers to two oxygen atoms bonded together, such as in the case of molecular oxygen (O2) gas.
In summary, one molecule of TiO2 contains two oxygen atoms. However, it's important to note that these oxygen atoms are not present as separate O2 molecules but are chemically bonded within the TiO2 compound.
To know more about molecules visit:
https://brainly.com/question/28931982
#SPJ11
Does the mass of an object always equal the sum of its parts?
Tree or false (No "I dont know" or "who knows" answers allowed)
Answers
Answer:
True
Explanation:
The mass of an object is always equal to the sum of its parts. ... Weight is a measure of the pull of gravity on an object's mass. The mass of an object is the same on Earth as it is on the moon, but the weight of the same object will be less on the moon than on Earth because there is less gravitational force on the moon.
. Sketch the nucleotide being described: it uses a monosaccharide present in RNA, and a nitrogenous base found only in RNA. Point an arrow to the glycosidic bond.
Answers
Answer:
See below
Explanation:
The glycosidic bond forms between the carbon atom on the glucose molecule and the nitrogen atom present on the nitrogenous base. A diagram has been attached to show this particular bond. There is also a phosphate molecule bonded on the sugar molecule at the other end.

How is the concentration of dye monitored during the reaction in this experiment? Select one: Measuring volume of gas produced O UV-Vis absorption O Redox Acid-base titration
Answers
The concentration of dye is monitored during the reaction in this experiment using UV-Vis absorption.
This technique involves measuring the absorption of light by the dye at a particular wavelength. As the dye concentration changes during the reaction, the amount of light absorbed also changes, which can be detected and used to determine the concentration of the dye.
This method involves passing light through the solution and measuring the absorbance of the specific wavelength corresponding to the dye. As the concentration of the dye changes during the reaction, the absorbance will change accordingly, allowing you to monitor the concentration throughout the experiment.
To know more about dye, refer
https://brainly.com/question/11225886
#SPJ11
What is the percent of chloride ion in a sample if 2. 500 g of the sample produces 1. 750 g of agcl when treated with excess ag⁺? report your answer to two decimal places.
Answers
The percent of chloride ion in a sample if 2. 500 g of the sample produces 1. 750 g of agcl when treated with excess ag⁺ is 17.2 percent.
What is mass percent?
The ratio of the mass of the solute contained in a solution to the mass of the solution as a whole is known as the mass percent.
Explanation:
Given:
Mass of sample = 2. 500 g
Mass of AgCl = 1. 750 g
The percent of chloride ion in a sample is calculated as,
Molar mass of AgCl = 143.32 g/mol
\(Molar mass of Chlorine atom = 35.45 g/mol\)
Assume that all of the sample's chlorine has precipitated into silver chloride. As a result, the amount of chlorine in silver chloride will match the amount of chlorine in the sample.
First we have to find mass of chlorine,
\(In 143.32 g of silver chloride, mass of chlorine present is 35.45 gSo, in 1.3487 g of silver chloride, mass of chlorine present will be =35.45g/143.32 g * 1.750 g = 0.43 g\)
Therefore, the percent of chloride ion in a sample is
\(Mass of pure chloride compound = 2.500 gMass of chlorine = 0.43 g%Chlorine = 0.43/2.5 *100%Chlorine = 17.2 %\)
Hence, the percent of chloride ion in a sample is \(17.2%\) percent.
To learn more about mass percentage from the given link.
https://brainly.com/question/17463660
#SPJ4
Suppose the isotopic ratio of the two boron isotopes 10B (10.013 amu) and 11B (11.009 amu) in a sample has been altered from the ratio found in nature and now contains 33.36% 10B in the sample. Determine the atomic weight of this sample of this new boron element.
Answers
Answer:
The correct answer is 10.676 amu.
Explanation:
Based on the given information, the concentration of 10B left in the sample is 33.36%. Therefore, the percentage of 11B present will be,
11B = 100% - 33.36% = 66.64%
Now the atomic weight of the new boron element can be determined by adding the atomic masses of both the isotopes multiplied by its percentage.
Therefore,
= (10.013 amu * 33.36%) + (11.009 amu * 66.64%) / 100
= 10.676 amu
PLEASE ANSWER QUICKLY!!!!! 50 POINTS
2KI(aq) + Cl₂(g) → 2KCI(aq) + 1₂ (g)
44.8 L 12 forms at STP. How many moles of KI were required for the
reaction?
?] mol KI
Answers
Answer:
It took about 3.74 moles of KI to complete the reaction.
The reaction between chlorine gas and potassium iodide is what kind?
Solid iodine (I2) and potassium chloride are produced when chlorine (Cl2) gas is passed through an aqueous solution of potassium iodide (KI) (KCl). This is a displacement reaction in which the more reactive chlorine displaces the less reactive iodine in potassium iodide.
n = PV/RT
where V is the volume, T is the temperature, and R is the gas constant. The temperature and pressure are both 1 atm at STP. By replacing these values, we obtain:
n = (1 atm) * (44.8 L) / (0.0821 L·atm/mol·K * 273 K) ≈ 1.87 mol I₂
We know that the number of moles of KI needed for the reaction is twice the number of moles of I2 produced since 1 mole of I2 is created for every 2 moles of KI.
n(KI) = 2 * n(I₂) ≈ 3.74 mol KI
Why ions move and in which direction they move in the presence of an electric field created between two electrodes having opposite charges
Answers
Answer and Explanation:
In an electric field generated between two electrodes there is a separation of charges: a negatively charged electrode called cathode and a positively charged electrode called anode. Ions are charged particles, so they can be positive or negative charges. Opposite charges attract each other. So, the cathode - negatively charged - attracts positively charged particles. On the other hand, the anode - positively charged - attracts negatively charged particles.
Therefore, positive ions move towards the cathode and negative ions move towards the anode.
How many gram of gaseous carbon dioxide can be absorbed or reacted by 2kg of lithium hydroxide?
Answers
Answer:
shuuuuuuuuuuuuuuuuuuuuuuush
Explanation:
shush suuush shuuush
What letter or pair of letters that represent the
element.
Answers
How many moles are in 7.46 x 1025 particles of iron
Answers
Answer:
1 mole of iron =6.023×10^23 particles
1 particles of iron=1/6.023×10^23 mole
7.46×10^25 particles =1/6.023×10^23×7.46×10^25
=1.238×10^48 mole is a required answer.
The final digit in a measurement is obtained by estimating between the smallestmarked lines.a) Trueb) False
Answers
Answer:
\(A:\text{ True}\)Explanation:
Here, we want to get how the final digit in a measurement is obtained
Mathematically, the final digit can be obtained by estimation
Hence, we say that the value is uncertain
The final digit is obtained by a mark or between the last mark and the next mark in a measurement
Thus, we call this value uncertain since it is estimated
Electrical energy is the energy of ___
Answers
Answer:Explanation:
Electrical energy is caused by moving electric charges called electrons. Electricity is a type of energy that comes from electrical energy. Kinetic energy is the energy of a moving object.
in the equation below what is the bronsted acid? HCL+NH3----- CL+NH4
Answers
\(\huge{{\mathfrak{Answer}{\color{black}{࿐}}}}\)
\(\longmapsto\)
In the Above equation, HCL is a bronsted acid, because it donated H+ ( one proton ) to NH3.
Question What does the law of conservation of mass state? Responses Mass can be created and destroyed during a chemical reaction. Mass can be created and destroyed during a chemical reaction. Mass can be created but not destroyed in a chemical reaction. Mass can be created but not destroyed in a chemical reaction. Mass cannot be created nor destroyed in a chemical reaction. Mass cannot be created nor destroyed in a chemical reaction. Mass can be destroyed but not created during a chemical reaction.\
Answers
According to the rule of conservation of mass, no atoms are created or destroyed during a chemical process. Instead, the atoms combine in a variety of ways to create goods.
What is law of conservation of mass ?According to the law of conservation of mass or principle of mass conservation, any system that is closed to all transfers of matter and energy must maintain a constant mass over time since the system's mass cannot vary and neither more nor less amount can be added or subtracted.
The law of conservation of mass, no atoms are created or destroyed during a chemical process. Instead, the atoms combine in a variety of ways to create goods. This is why each element has the same amount of atoms on both sides of an equation with balanced symbols.
Thus, According to the law of conservation of mass, mass is neither generated nor destroyed during a chemical process.
To learn more about law of conservation of mass refer the link below;
https://brainly.com/question/20635180
#SPJ1
Thor pushes a car with a mass of 2500 kg. It accelerates 5 m/s2. How much force did Thor apply?
Answers
Answer:
The answer is 12500 NExplanation:
The force acting on an object given it's mass and acceleration can be found by using the formula
force = mass × acceleration
From the question we have
force = 2500 × 5
We have the final answer as
12500 NHope this helps you
nuclear decay occurs according to first-order kinetics. a nuclide decays in 3.40 days from 45.0 g to 12.1 g. what is the rate constant for the nuclide?
Answers
Its rate constant again for nuclide is 0.388 days per one. First-order kinetics governs how nuclear decay happens. At 45.0 g down 12.1 g, a nuclide degrades every 3.40 days.
How do you define a nuclide?Nuclide, also known as nuclear species, is an atom species that is identified by the amount of protons, neutrons, and the energetic state of the nucleus. As a result, a nuclide is identified by its quantity (A) and atomic number (Z).
What is an example of a nuclide?A similar options is a type of atom that has a certain ratio of protons to neutrons in its nucleus, such as carbon-13's 6 protons to 7 neutrons.
to know more about nuclide visit:
https://brainly.com/question/9826865
#SPJ4
Based on the periodic table, what is the oxidation number of oxygen (Group VI, period 2) in compounds? a) 0 b) +2 C) -2 d) 1
Answers
The oxidation number of oxygen (Group VI, period 2) is -2.
The electrical charge of an atom describes its Oxidation number.\The number of electrons that must be gained or lost in an atom's valence electron shell to make it to be filled or half-filled gives us the oxidation number value.When the atom gains electrons,it acquires a negative oxidation number. When the atom loses electrons,it acquires a positive oxidation number
Oxygen has 8 electrons with {He] 2s² 2p⁴ configuration. With 6 electrons in its valence shell, it need to gain 2 electrons to achieve stability, When it gains 2 electrons, it acquires a negative oxidation number of -2.
See more here:https://brainly.com/question/11621135
A pi bond is the result of the a) overlap of two s orbitals. b) overlap of an s orbital and a p orbital. c) overlap of two p orbitals along their axes. d) sideways overlap of two parallel p orbitals. e) sideways overlap of two s orbitals.
Answers
A pi bond is the result of the d) sideways overlap of two parallel p orbitals.
Pi bonds are bonds that occur as a result of overlapping orbitals of atoms that are not in the bond axis. Each p orbital that contributes to a pi bond has two lobes and has a node at the core.
The pi orbital can hold a maximum of two pairs of electrons. Whereas each electron in a pi bond is also called a pi electron, the pi electrons are used for double bonds or triple bonds. The 2p orbital of carbon has slightly higher energy than the sp2 orbital, so the pi bond formed from two 2p orbitals has somewhat higher energy and is slightly less stable than the sp2-sp2 sigma bond.
Learn more about pi bonds at:
https://brainly.com/question/13243902
#SPJ4