Answers
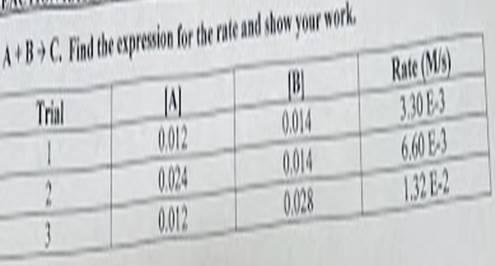
The reaction A + B → C has the following rate expression is 197.62 [A][B] M/s
How to determine rate expression?Using the experimental data to determine the order of the reaction with respect to A and B, assume that the rate of the reaction is given by:
rate = \(k[A]^x[B]^y\)
where k = rate constant and
x and y = orders of the reaction with respect to A and B, respectively.
Compare the rates of the reaction in trials 1 and 2 while keeping the concentration of A constant:
rate1/rate2 = \(\frac{k[A]^x[B]^y}{k[A]^x[B]^y} = \frac{[B]^y}{[B]^y} = 1\)
Conclude that the reaction is first-order with respect to B.
Similarly, compare the rates of the reaction in trials 1 and 3 while keeping the concentration of B constant:
rate1/rate3 =\(\frac{k[A]^x[B]^y}{k[A]^x[B]^y} = \frac{[A]^x}{[A]^x} = 1\)
Therefore, the reaction is first-order with respect to A.
The rate expression for the reaction A + B → C is:
rate = k[A][B]
Using any of the experimental trials to determine the value of the rate constant k, use trial 1:
rate1 =\(k[A]^1[B]^1\)
k = \(\frac{rate1}{[A]^1[B]^1}\) = (3.30 E-3)/(0.012 M x 0.014 M) = 197.62 M⁻² s⁻¹
Therefore, the rate expression for the reaction A + B → C is:
rate = 197.62 [A][B] M/s
In this case, the units of k are M⁻¹ s⁻¹ because the reaction is first-order with respect to both A and B.
Find out more on rate expression here: https://brainly.com/question/15154019
#SPJ1
Related Questions
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.
how we can remove temporary hardness of water?and write the chemical reaction?
Answers
Answer:
it can be removed
1. by boiling
Ca(HCO3)2 > CaCO3 + H2O +CO2
2. by treating with calcium hydroxide
What can you infer is causing the cryosphere to change?
Answers
Answer:
Explanation:
The cryosphere—the portion of the Earth's surface where water is in solid form for at least one month of the year—has been shrinking in response to climate warming. ... The important outcome, however, is the change and the response the human social system (infrastructure, food, water, recreation) will have to that change.
The cryosphere the portion of the Earth's surface where water is in solid form for at least one month of the year has been shrinking in response to climate warming.
What is cryosphere ?The cryosphere includes all of the earth's frozen water, including glaciers and sea ice.
The lithosphere is the solid part of the earth made up of rocks, minerals, and other elements except the ocean. It is the part which present in the outer part of the earth which includes the brittle upper portion of the mantle and crust. The part of planet where living things can be found from the upper part of the atmosphere to the lower of the oceans and pools that are thermal and made up of the parts of earth where life present in all ecosystem is known as biosphere.
The atmosphere is the thin layer of gases that covers the outer part of the planet. It gives us the many different climates, or broad way of weather conditions around the world. The hydrosphere includes all of the water on earth from the oceans to glaciers and underground water and it can be liquid, vapour, or ice.
Therefore, the cryosphere the portion of the Earth's surface where water is in solid form for at least one month of the year has been shrinking in response to climate warming.
Learn more about cryosphere here:
https://brainly.com/question/10258472
#SPJ2
Alka was making tea in a kettle. Suddenly she felt intense heat from the puff of steam gushing out of the spout of the kettle. She wondered whether the temperature of the steam was higher than that of the water boiling in the kettle. Comment. (2)
Answers
It is likely that the temperature of the steam is higher than the temperature of the water boiling in the kettle. The intense heat felt by Alka from the puff of steam supports this observation.
In general, the temperature of steam produced from boiling water is higher than the temperature of the water itself. When water boils, it undergoes a phase change from a liquid to a gas, forming steam.
During this phase change, the water absorbs heat energy from the heat source, such as a stove or electric kettle, and converts it into the latent heat of vaporization.
The boiling point of water is 100 degrees Celsius (212 degrees Fahrenheit) at standard atmospheric pressure. At this temperature, the water molecules have enough energy to overcome the intermolecular forces and transition into the gaseous state.
However, steam is hotter than the boiling point of water because it contains additional heat energy in the form of latent heat. The heat energy absorbed during vaporization is stored as latent heat within the steam. As the steam gushes out of the spout of the kettle, it releases this latent heat energy, which can be felt as intense heat.
For more question on temperature click on
https://brainly.com/question/4735135
#SPJ11
What is the chemical name
for ca2 and clo3-
Answers
Answer:
Calcium chlorate | Ca(ClO3)2 - PubChem.
Explanation:
Hydrogen gas is collected over water at 23°C, 767 torr. At this temperature the vapor pressure of water is 21.0 torr. What is the partial pressure of hydrogen in the collected gas?
Answers
The partial pressure of hydrogen gas in the collected gas is 746 torr.
To determine the partial pressure of hydrogen gas in the collected gas, we need to consider the difference between the total pressure of the collected gas and the vapor pressure of water at the given temperature. The partial pressure of hydrogen gas is the pressure exerted by hydrogen alone.
Given information:
Total pressure of the collected gas (Ptotal) = 767 torr
Vapor pressure of water (Pwater) = 21.0 torr
The partial pressure of hydrogen gas (Phydrogen) can be calculated using Dalton's law of partial pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each individual gas.
Phydrogen = Ptotal - Pwater
Plugging in the given values:
Phydrogen = 767 torr - 21.0 torr
Phydrogen = 746 torr
Therefore, the partial pressure of hydrogen gas in the collected gas is 746 torr.
It's important to note that in this calculation, we assume that the water vapor does not react with or dissolve in the hydrogen gas and that the gases behave ideally. Additionally, it's assumed that the collected gas is dry, meaning all the water vapor has been removed or does not significantly contribute to the total pressure.
Fo rmore such questions on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
GIVING BRAINLIEST I NEED ANSWER IN LESS THAN 5 mins!
Is this a scientific model? Use complete sentences to explain why or why not.

Answers
Answer:
its a scientific model
Explanation:
It is a scientific model because it is showing the water cycle. In the water cycle it is scientifically showing how water circulates through the Earth. For example, the water in the ocean evaporates which is evaporation, then it condensates into a cloud that then becomes condensation and the condensation makes precipitation when it rains. When it falls to the ground, it then becomes a runoff down a hill.
Predict the total pressure in Container C if the initial pressure in Container A was doubled and Container B was reduced by one-half, then mixed in Container C. Show your work.
Answers
The total pressure in Container C is \(5_{P}\)/(\(2_{V}\)). If the initial pressure in Container A was doubled and Container B was reduced by one-half.
To solve this problem, we need to use combined gas law, which relates with pressure, volume, and temperature of a gas;
(P₁V₁)/T₁ = (P₂V₂)/T₂
where P₁ and V₁ are initial pressure and volume, respectively, and T₁ is initial temperature. Similarly, P₂, V₂, and T₂ are inal pressure, volume, and temperature, respectively.
Let's assume that the volume and temperature are constant in all three containers. Therefore, we can simplify the equation to;
P₁/P₂ = V₁/V₂
We can use this equation to solve for the final pressure in Container C.
First, let's calculate the new pressures in Containers A and B;
Container A; the initial pressure was doubled, so P₁ = \(2_{P}\) and V₁ = V (since the volume is constant). Therefore, P₂ = P₁/(V₁/V₂) = \(2_{P}\)/(1/2) = \(4_{P}\).
Container B; the initial pressure was reduced by one-half, so P₁ = P/2 and V₁ = V (since the volume is constant). Therefore, P₂ = P₁/(V₁/V₂) = (P/2)/(1/2) = P.
Now that we have the new pressures in Containers A and B, we can use them to find the total pressure in Container C:
Container C; we are mixing equal volumes of gases from Containers A and B, so the total volume is \(2_{V}\). The total pressure is the sum of the partial pressures of the gases in Containers A and B, which are \(4_{P}\) and P, respectively. Therefore, the total pressure in Container C is:
\(P_{total}\) = (\(4_{P}\) + P)/(\(2_{V}\))
= \(5_{P}\)/(\(2_{V}\))
So, the final pressure in Container C is \(5_{P}\)/(\(2_{V}\)).
To know more about combined gas law here
https://brainly.com/question/13154969
#SPJ1
2NO(g) + O₂(g) = 2NO₂(g)
AH = -112 kJ K = 0.50
The equilibrium concentrations are
[NO] = 0.31 M, [02] = 1.10 M, and
[NO2] = [?]
What is the equilibrium concentration of
NO2 at this temperature?
![2NO(g) + O(g) = 2NO(g)AH = -112 kJ K = 0.50The equilibrium concentrations are[NO] = 0.31 M, [02] = 1.10](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/YRGcrEmw6OtcUiE6tB5KUryscULOSlX5.png)
Answers
The equilibrium concentration of NO₂ at this temperature is approximately 0.219 M.
To determine the equilibrium concentration of NO₂, we can use the equilibrium constant expression (Kc) and the given equilibrium concentrations of NO and O₂. The equilibrium constant expression for the given reaction is:
Kc = ([NO₂]²) / ([NO]²[O₂])
We are given the equilibrium concentrations of NO and O₂ as [NO] = 0.31 M and [O₂] = 1.10 M, respectively. We need to find the equilibrium concentration of NO₂, denoted as [NO₂].
Using the given equilibrium concentrations and the equilibrium constant expression, we can rearrange the equation and solve for [NO₂]:
Kc = ([NO₂]²) / ([NO]²[O₂])
0.50 = ([NO₂]²) / ((0.31 M)²(1.10 M))
0.50 = ([NO₂]²) / (0.0961 M³)
Multiplying both sides by 0.0961 M³, we have:
0.04805 M³ = [NO₂]²
Taking the square root of both sides, we find:
[NO₂] = √(0.04805 M³)
[NO₂] ≈ 0.219 M
Therefore, the equilibrium concentration of NO₂ at this temperature is approximately 0.219 M.
It's important to note that the units of concentration (M) were used throughout the calculations, and the answer is rounded to three significant figures based on the given data.
Additionally, the negative sign of the enthalpy change (AH) indicates an exothermic reaction, and the equilibrium constant (K) of 0.50 suggests that the reaction favors the products, as the concentration of NO₂ is greater at equilibrium.
For more such questions on equilibrium concentration visit:
https://brainly.com/question/13414142
#SPJ8
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
two uses of sodium carbonate
Answers
Sodium carbonate, also known as washing soda or soda ash, has a wide range of applications. Sodium carbonate can be naturally occurring or synthetically produced through various methods, including the Solvay process, which is the most common method of industrial production.
Sodium carbonate, also known as washing soda or soda ash, has many uses, including:
1) Cleaning agent: Sodium carbonate is an effective cleaning agent due to its alkaline nature. It is used in laundry detergents and household cleaners to remove stains and grease from clothes and surfaces.
2) Industrial applications: Sodium carbonate is used in a variety of industrial applications. It is used in the production of glass, pulp and paper, and soaps and detergents. It is also used as a water softener and pH regulator in chemical processes.
Learn more about Sodium Carbonate at
brainly.com/question/31344166
#SPJ1
Choose the equation below that is balanced correctly.
S8 +24 028 SO3
S8+ 12 0₂8 SO3
6 S8+8 026 SO3
2 S8 +3 022 SO3
Answers
The balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is 2S₈ + 16O₂ → 16SO₃.
What is the balanced chemical equation?Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products.
The balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is determined as;
2S₈ + 16O₂ → 16SO₃
From the reactants side we can see that sulfur is 16 and also 16 in the product side. The number of oxygen in the reactant side is 32 and also 32 in the product side.
Thus, the balanced equation for the reaction between sulfur (S₈) and oxygen (O₂) to form sulfur trioxide (SO₃) is 2S₈ + 16O₂ → 16SO₃.
Learn more about balanced chemical equation here: https://brainly.com/question/26694427
#SPJ1
Compare the way that frogs breathe when they are tadpoles with the way frogs breathe when they are adults.
Answers
Answer:Frogs breathe through gills underwater to get oxygen and when frogs are adults they breathe through their lungs to get oxygen.
Explanation:
Which products are produced when a mixture of propanol and concentrated potassium dichromate is heated?
Answers
The products produced when a mixture of propanol and concentrated potassium dichromate is heated is propionaldehydeCH3CH2CHO + Cr2(SO4)3 + K2SO4 + H2O
Products of chemical reaction explained.
A chemical reaction is a process that leads to the transformation of one or more substances into different substances with different properties. During a chemical reaction, the atoms of the reactants rearrange themselves into new arrangements, resulting in the formation of one or more new substances called products.
When a mixture of propanol (specifically, 1-propanol) and concentrated potassium dichromate is heated, the primary product that is formed is propionaldehyde. This is an oxidation reaction where the potassium dichromate acts as an oxidizing agent, causing the alcohol functional group (-OH) on the propanol to be converted into an aldehyde functional group (-CHO).
The chemical equation for this reaction is:
CH3CH2CH2OH + K2Cr2O7 + H2SO4 → CH3CH2CHO + Cr2(SO4)3 + K2SO4 + H2O
In addition to propionaldehyde, small amounts of other oxidation products such as acetone and acetic acid may also be formed, depending on the reaction conditions.
Learn more about chemical reaction below.
https: //brainly.com/question/11231920
#SPJ1
Calculate the bond energy of the Br-Cl bond, in kJ/mol, using AHº for the reaction (1.6 kJ/mol) and the information in the following table Bond Bond Energy (kJ/mol)
Br - Br 193
CI - CI 243
Br - CI ?
Answers
The bond energy of the Br-Cl bond would be 217.2 KJ/mol.
\(Cl_{2}\) + \(Br_{2}\) ----> 2 Br - Cl
Δ H° reaction = (bond of energy of \(Cl_{2}\) + bond energy of \(Br_{2}\) ) - 2 (bond energy of Br - Cl)
1.6 = 243 + 193 - 2 (B.E of Br - Cl)
Bond energy of Br - Cl = 243 + 193 - 1.6 / 2 = 217.2 KJ/mol
Electrostatic forces between negatively charged electrons and positively charged atomic nuclei produce bonds. The amount of energy required to separate the atoms forming a molecular bond into free atoms is known as bond energy, and it serves as a gauge of the strength of a chemical connection.
To learn more about bond energy
https://brainly.com/question/26141360
#SPJ4
Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
Ca(OH)2 + 2HNO3 → Ca(NO3)2 + 2H2O
Answers
Answer: You have it right
Explanation:
you put the two on the H2O to make it 2H2O and A two on the HNO3 to make it 2HN03 To make it balanced, good job
Calculate the molar mass of the following:
1. HCI
2. ZnF2
3. K2CO3
4. FePO4
5. Cu2s
Show all your work please thank you

Answers
Answer:
1. 36.5g 2. 103.3g
3. 140g 4. 151g
5. 159g
Explanation:
1. HCl
atomic mass of H= 1g
atomic mass of Cl = 35.5g
solution:
HCl
= 1+ 35.5
= 36.5g
2. ZnF2
atomic mass of Zn = 65.3g
atomic mass of F = 19g
solution:
ZnF2
= 65.3 + 2(19)
=65.3 + 38
= 103.3 g
3. K2CO3
atomic mass of K= 40g
atomic mass of C = 12g
atomic mass of O = 16g
Solution:
K2CO3
= 2(40) + 12 + 3 (16)
= 80 + 12 + 48
= 140g
4. FePO4
atomic mass of Fe = 56g
atomic mass of P= 31 g
atomic mass of O = 16g
solution:
FePO4
= 56 + 31 + 4 (16)
= 56 + 31 + 64
= 151g
5. Cu2S
atomic mass of Cu = 63.5g
atomic mass of S = 32 g
solution
Cu2S
= 2(63.5 )+ 32
= 127 + 32
=159g
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
How many moles of H20 are required to react completely with 7.30 moles of NO2?
3NO2(g) + H200 +
NO(g) + 2HNO3(aq) A
1.86 mol
2.19 mol
2.43 mol
O 6.12 mol
O 7.30 mol
Answers
Answer:
2.43 moles of water are require.
Explanation:
Number of moles of water required = ?
Number of moles of NO₂ available = 7.30 mol
Solution:
Chemical equation:
3NO₂ + H₂O → NO + 2HNO₃
now we will compare the moles of NO₂ with H₂O.
NO₂ : H₂O
3 : 1
7.30 : 1/3×7.30 = 2.43 mol
A balloon holds 60.0 kg of helium. What is the volume of the balloon if the final pressure is 1.20 atm and the temperature is 22°C?
Answers
Answer:
Explanation:
Use the formula PV=nRT
P is pressure in atm
V is volume in whatever unit you're working in as long as everything is in that unit (anything volume related)
n is the number of moles
R is the constant so 0.08206
and T is temperature and this MUST be in Kelvin which is 173.15 + C
the equation can be shifted depending on what you need to solve
Explain how these results show that chlorine is more reactive than bromine and
lodine.
Answers
Chlorine is more reactive than bromine because it replaces both bromine and iodine.
How chlorine is more reactive than bromine?Fluorine is the most sensitive while on the other hand, the astatine is the least reactive as compared to other elements. The chlorine displaces both bromine and iodine, and bromine displaces iodine because of its high reactivity. The element that replaces other atom is considered as more reactive.
The order of reactivity is that the chlorine is more reactive than bromine, which indicates that chlorine is more reactive than iodine.
So we can conclude that chlorine is more reactive than bromine due to high reactivity.
Learn more about Chlorine here: https://brainly.com/question/24218286
#SPJ1
Which best defines color? (1 point)
a physical property of matter related to how a material interacts with different wavelengths
of light
a chemical property of matter related to how a material interacts with different wavelengths
of light
O a physical property of matter related to a material's ability to reflect light
O a chemical property of matter related to a material's ability to reflect light
Answers
Answer:
i believe the answer to your quetion will be A im on edge by the way so if your on a differnt program than it might be wrong sorry if im wrong have a wonderfull day!
How many mL of a 5.00% (m/v) glucose solution will be needed to deliver 8.5 grams of glucose?
Answers
mass = volume x concentration x density
where mass is the amount of glucose needed, volume is the volume of the glucose solution we need to prepare, concentration is the percentage of glucose in the solution, and density is the density of the solution.
We can rearrange the formula to solve for volume:
volume = mass / (concentration x density)
The density of the 5.00% (m/v) glucose solution can be assumed to be 1.00 g/mL.
Plugging in the values, we get:
volume = 8.5 g / (5.00 g/100 mL x 1.00 g/mL) = 170 mL
Therefore, we need 170 mL of the 5.00% (m/v) glucose solution to deliver 8.5 grams of glucose.
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
A compound with a molecular mass of 44.0 grams is found to be 81.82% carbon
and 18.18% hydrogen by mass. Finds its molecular formula. (HINT: Once you get
the mole to mole ratio you will need to multiple both by 3)
Answers
The molecular formula of the compound containing 81.82% carbon
and 18.18% hydrogen by mass is C₃H₈
From the question given above, the following data were obtained:
Carbon (C) = 81.82%
Hydrogen (H) = 18.18%
Molar mass of compound = 44 g/mol
Molecular formula =?We'll begin by calculating the empirical formula of the compound. This can be obtained as follow:
Carbon (C) = 81.82%
Hydrogen (H) = 18.18%
Empirical formula =?Divide by their molar mass
C = 81.82 / 12 = 6.818
H = 18.18 / 1 = 18.18
Divide by the smallest
C = 6.818 / 6.818 = 1
H = 18.18 / 6.818 = 2.67
Multiply by 3 to express in whole number
C = 1 × 3 = 3
H = 2.67 × 3 = 8
Thus, the empirical formula of the compound is C₃H₈
Finally, we shall determine the molecular formula of the compound. This can be obtained as follow:
Molecular formula = Empirical formula × n = molar mass
[C₃H₈]n = 44
[(12×3) + (1×4)]n = 44
[36 + 4]n = 44
40n = 44
Divide both side by 40
n = 44/40
n ≈ 1
Molecular formula = C₃H₈ × n
Molecular formula = C₃H₈ × 1
Molecular formula = C₃H₈Therefore, the molecular formula of the compound is C₃H₈
Learn more: https://brainly.com/question/13208888
Rearrange the equation to isolate . =
If =8.00 , =9.00 , and =3.00 , what is the value of ?
Answers
Answer:
Explanation:
8.00 + 9.00 + 3.00 = 20 i dont really know what your asking but i bet your really smart!
A solution of ethanol (C2H6O) in water is sometimes used as a disinfectant. 1.00 L of this solution contains 553 g of ethanol and 335 g of water. What is the molality of the ethanol in this solution?
Answers
Answer:
The molality of a solution can be calculated by dividing the number of moles of solute (ethanol) by the mass of the solvent (water) in kilograms. To calculate the moles of ethanol, we need to know its molar mass:
C2H6O (ethanol) molar mass = 2(12.01 g/mol) + 6(1.01 g/mol) + 16.00 g/mol = 46.07 g/mol
Next, divide the total mass of ethanol (553 g) by its molar mass (46.07 g/mol) to get the number of moles:
553 g / 46.07 g/mol = 12.0 mol
Next, convert the mass of water (335 g) to kilograms:
335 g / 1000 g/kg = 0.335 kg
Finally, divide the number of moles of ethanol (12.0 mol) by the mass of water in kilograms (0.335 kg) to get the molality:
12.0 mol / 0.335 kg = 35.8 mol/kg (to 3 significant figures)