chemical signals that have been processed and integrated are sent to this part of the neuron called
Answers
Chemical signals that have been processed and integrated are sent to the axon hillock of the neuron.
Let's understand this in detail:
What is a neuron?
Neurons are specialized cells capable of transmitting electrical and chemical signals in response to stimuli. The axon hillock is located at the base of the axon, where signals are integrated before being transmitted down the axon.
When a neuron receives multiple signals, they are processed and integrated at the axon hillock before being transmitted down the axon to the synapse for further communication with other neurons.
Therefore, chemical signals that have been processed and integrated are sent to the axon hillock of the neuron.
#SPJ11
Learn more about neurons: Name three types of neurons and give the function of each. https://brainly.com/question/13061744
Related Questions
The isotope uranium-238 decays at a constant rate (half-life of 8.2 x 1015 years). It also occasionally divides in half, and in this process of fission the two parts are violently repelled from one another, creating track-like scars in crystalline materials and obsidian. By counting the frequency of fission tracks and comparing this to the known rate for fission, the archaeologist can calculate the age of the sample. This dating technique is called
Answers
The dating technique described in your question, which involves counting the frequency of fission tracks in a sample and comparing it to the known rate of fission, is called fission track dating.
This method relies on the decay of uranium-238, which has a half-life of 8.2 x 10¹⁵ years.
When uranium-238 undergoes fission, the resulting fragments are violently repelled from each other, leaving behind track-like scars in materials such as crystalline substances and obsidian.
By measuring the number of fission tracks in a sample and knowing the rate of fission, archaeologists can estimate the age of the sample.
Fission track dating has been used to determine the ages of various geological and archaeological materials.
To know more about fission visit:
https://brainly.com/question/82412
#SPJ11
Perform the following operation and express the answer in scientific notation 8.6500x10^3+6.5500x10^5
Answers
Taking into account the scientific notation, the result of the sum is 6.6365×10⁵.
Scientific notationFirst, remember that scientific notation is a quick way to represent a number using powers of base ten.
The numbers are written as a product:
a×10ⁿ
where:
a is a real number greater than or equal to 1 and less than 10, to which a decimal point is added after the first digit if it is a non-integer number.n is an integer, which is called an exponent or an order of magnitude. Represents the number of times the comma is shifted. It is always an integer, positive if it is shifted to the left, negative if it is shifted to the right.Sum in scientific notationYou want to add two numbers in scientific notation. It should be noted that when the numbers to be added do not have the same base 10 exponent, the base 10 power with the highest exponent must be found.
In this case, the highest exponent is 5.
Then all the values are expressed as a function of the base 10 exponent with the highest exponent. In this case: 8.6500×10³= 0.086500×10⁵
Taking the quantities to the same exponent, all you have to do is add what was previously called the number "a". In this case:
0.086500×10⁵ + 6.5500×10⁵= (0.086500+ 6.5500)×10⁵= 6.6365×10⁵
Finally, the result of the sum is 6.6365×10⁵.
Learn more about scientific notation:
brainly.com/question/11403716
brainly.com/question/853571
#SPJ1
In a 2.00 g sample, which has the greatest number of atoms?
AI
S
All are equal
Na
Р
Answers
Answer
so AI has 13 atoms
S has 16 atoms
Na has 11 atoms
P has has 15 atoms
so the one with the most is S
S is sulfur
sulfur has many uses when it is processed along with some some biological uses.
Explanation:
Name the layer where the pressure is 3.5 million atmospheres:
Answers
Answer:
The Inner Core
hope it was useful
stay at home stay safe
keep rocking
pls mark me as brani
........
PLS HELP MARKING BRAINLIEST! (Dont answer if u dont know and please don't answer random things-)
What happens when Mentos and Diet Coke are mixed?
What causes Mentos and Diet Coke to react that way?
Is the reaction Chemical or Physical?
How could we prove one way or the other?
Answers
When mentos and diet coke are mixed, the diet soda explodes
Explanation:
Due to the fact that the mentos are dense, they sink to the bottom of the bottle which gives the diet coke its explosion.
Answers & Explanations:
What happens when Mentos and Diet Coke are mixed?
Mentos sinks rapidly through the liquid, causing a fast, large eruption.
What causes Mentos and Diet Coke to react that way?
The addition of the Mentos leads to the rapid nucleation of carbon dioxide gas bubbles in the Diet Coke, causing them to precipitate out of the solution.
Is the reaction Chemical or Physical?
The eruption is caused by a physical reaction.
How could we prove one way or the other?
We can prove it by using the equation:
\(CO_{2} (aq) = Co_{2}(g)\)
Explain how the complexity and diversity of living things has changed over time.
Answers
Answer:A complex biological structure with many interacting parts might appear, at first glance, as if it were originally created in its present form with all its interlocking components fully formed and intact. It doesn’t seem possible that they developed step by step via biological evolution. In Darwin’s Black Box, Michael Behe introduces a term that he and other proponents of Intelligent Design use for this concept: irreducible complexity. No part of an irreducibly complex system has any apparent function except in its relation to the other parts.
Can anyone please answer questions 3 and 4
I’ll give the brainiest!
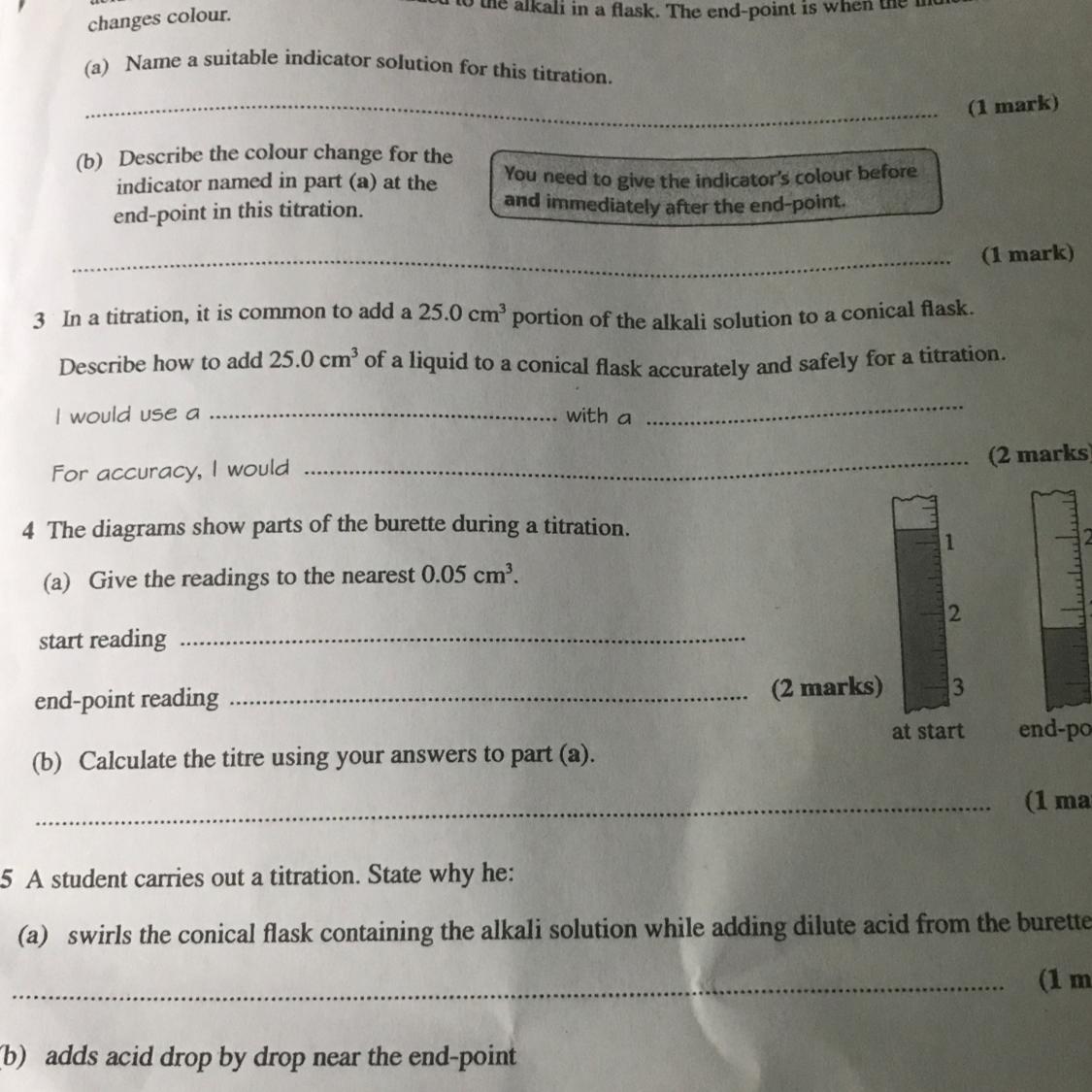
Answers
Answer:
1.Phenolphthalein
2.Method
a.Use the pipette and pipette filler to add 25 cm 3 of alkali to a clean conical flask.
b.Add a few drops of indicator and put the conical flask on a white tile.
c.Fill the burette with acid and note the starting volume.
d.Slowly add the acid from the burette to the alkali in the conical flask, swirling to mix.
Explanation:
Express solution concentration in terms of molality.
Answers
Explanation:
Molality of a solution is the number of moles of solute dissolved in 1 Kg of the solvent. Thus, if one gram molecule of a solute is present in 1 kg of the solvent.
hope this helps you
have a great day :)
The following symbol (<-->) means
Answers
Answer:
Greater than or less than
Please help me with these problems asap

Answers
1. The new volume would be approximately 0.082 L. for 2, The new pressure would be approximately 135.7 kPa. for 3, The new temperature would be approximately 223.3 K. for 4, The new temperature would be approximately 377.3 K. for 5, The new volume would be approximately 0.07 L.
1, Given: P₁ = 202 kPa, V₁ (volume)= 100 mL = 0.1 L, T₁ (temperature )= 25°C = 298 K ,P₂ = 250 kPa ,T₂ = 35°C = 308 K
Using the combined gas law formula, one can rearrange it to solve for V₂: V₂ = (P₁V₁T₂) / (P₂T₁)
V₂ = (202 kPa × 0.1 L × 308 K) / (250 kPa × 298 K)
V₂ ≈ 0.082 L
The new volume would be approximately 0.082 L.
2, Given: V₁ = 150 mL = 0.15 L ,T₁ = 100°C = 373 K ,P₁ = 303.9 kPa, T₂ = 50°C = 323 K ,V₂ = 225 mL = 0.225 L
Again, using the combined gas law formula and solving for P₂: P₂ = (P₁V₁T₂) / (V₂T₁)
P₂ = (303.9 kPa × 0.15 L × 323 K) / (0.225 L × 373 K)
P₂ ≈ 135.7 kPa
The new pressure would be approximately 135.7 kPa.
3, Given: V₁ = 500 mL = 0.5 L, T₁ = 0°C = 273 K (STP) ,P₁ = 101.3 kPa (STP) ,V₂ = 0.5 * V₁ = 0.25 L, P₂ = 2 * P₁ = 2 * 101.3 kPa = 202.6 kPa
To determine the new temperature, we rearrange the formula to solve for T₂:
T₂ = (P₂V₂T₁) / (P₁V₁)
T₂ = (202.6 kPa× 0.25 L ×273 K) / (101.3 kPa ×0.5 L)
T₂ ≈ 223.3 K
The new temperature would be approximately 223.3 K.
4, Given: V₁ = 150 mL = 0.15 L, P₁ = 50 kPa ,T₁ = 10°C = 283 K, P₂ = 200 kPa ,V₂ = 200 mL = 0.2 L
Solving for T₂:
T₂ = (P₂V₂T₁) / (P₁V₁)
T₂ = (200 kPa × 0.2 L × 283 K) / (50 kPa ×0.15 L)
T₂ ≈ 377.3 K
The new temperature would be approximately 377.3 K.
5, Given: V₁ = 150 mL = 0.15 L ,T₁ = 100°C = 373 K, P₁ = 500 kPa ,T₂ = 0°C = 273 K (STP) ,P₂ = 101.3 kPa (STP)
To determine the new volume, we rearrange the formula to solve for V₂:
V₂ = (P₁V₁T₂) / (P₂T₁)
V₂ = (500 kPa × 0.15 L × 273 K) / (101.3 kPa × 373 K)
V₂ ≈ 0.07 L
The new volume would be approximately 0.07 L.
Learn more about the gas law here.
https://brainly.com/question/30458409
#SPJ1
Why were your results for the Southern Hemisphere opposite of what you found for
the Northern Hemisphere?
Answers
Caption for this picture asap.

Answers
Answer:
1.conical flask
2.beaker
3 graduated cylinder
4.receiver
5.distillation flask
Explanation:
the name of material is this!
Each element emits its own unique wavelength of light which can be used to determine the identity of that element. The packet or light wave emitted is called a
Answers
Answer:
Photon
Explanation:
The simplest description of a photon is to consider it as a bundle of electromagnetic energy.
In the context of wave - particle duality, light is considered sometimes as a particle. Each bundle of the particles of light wave is called a photon of light.
The wavelength of a photon of light emitted by an element can be used as a tool to identify that particular element.
b) Sodium + Oxygen Sodium Oxide + Type of Reaction :
c) Barium Chloride + Potassium iodide Barium lodide + Potassium Chloride Type of Reaction :
e) NH 3 +HCl NH 4 Cl Type of Reaction ;
f) CuO+H 2 Cu+H 2
O Type of Reaction
g) CaOH CaO 2 +H 2 O Type of Reaction
Answers
Answer:
b. combustion reaction
c. displacement reaction
e. Decomposition reaction
f. redox reactions
g. combination reaction
I'm not fully sure about part c but the rest are correct
I hope it helped
What is the quickest method to find the number of resonating structures of O3, and SO4-2 like? (within 30 seconds) The best answer will make the brainiest
Answers
To quickly find the number of resonating structures of O3 and SO4-2, you can use the formula:
Number of resonating structures = 2^(number of equivalent resonance structures)
For O3, each oxygen atom is equivalent and there are two possible equivalent resonance structures:
O = O - O and O - O = O
Therefore, the number of resonating structures for O3 is:
2^(2) = 4
For SO4-2, there are two equivalent resonance structures for the sulfur-oxygen bonds and four equivalent resonance structures for the sulfate ion as a whole:
S = O and S - O(-)
O(-) - S - O and O = S = O(-)
Therefore, the number of resonating structures for SO4-2 is:
2^(2) x 2^(4) = 16
So the number of resonating structures for O3 is 4, and the number of resonating structures for SO4-2 is 16.
What volume of 0. 900% w/v saline solution can be prepared from 0. 300 L of a 3. 00% w/v saline solution available in stock?
Answers
The volume of 0.900% w/v saline solution that can be prepared from 0.300 L of a 3.00% w/v saline solution available in stock is 1.00 L.
To prepare a 0.900% w/v saline solution from a 3.00% w/v stock solution, we need to use the dilution formula:
C₁V₁ = C₂V₂
where C₁ is the concentration of the stock solution, V₁ is the volume of the stock solution, C₂ is the concentration of the diluted solution, and V₂ is the volume of the diluted solution.
Plugging in the given values, we get:
(3.00%)(0.300 L) = (0.900%)(V₂)
Solving for V₂, we get:
V₂ = (3.00%)(0.300 L) / (0.900%)
V₂ = 1.00 L
Therefore, the volume of the 0.900% w/v saline solution that can be prepared from the 0.300 L of the 3.00% w/v stock solution is 1.00 L.
Learn more about dilution here: https://brainly.com/question/24697661.
#SPJ11
A ____________ is a property, the expansion, redevelopment, or
reuse of which may be complicated by the presence or potential
presence of a hazardous substance, pollutant, or contaminant.
Answers
A brownfield is a property, the expansion, redevelopment, or reuse of which may be complicated by the presence or potential presence of a hazardous substance, pollutant, or contaminant.
A “brownfield” generally refers to a parcel of land that was previously used for industrial purposes and which is contaminated by low concentrations of hazardous chemicals.
A brownfield development requires more work and investment upfront: existing structures may have to be demolished, materials must be removed, and developers may have to engage in extensive environmental cleanup to remove pollutants.
Learn more about Brownfield land, here:
https://brainly.com/question/3762221
#SPJ4
This is how osmium appears in the periodic table.A purple box has O s at the center and 76 above. Below it says osmium and below that 190.23.
Rounded to the nearest whole number, how many neutrons, on average, are in an atom of osmium?
a76
b114
c 190
d266
Answers
Answer:
114
Explanation:
plz make me brainiest
We are studying the ideal gas law. In this discussion, you will be trying your hand at applying one of the ideal gas laws to a real world situation. Consider a situation that involves an ideal gas law and discuss how you would apply your chosen ideal gas law to the situation. Generate an ideal gas law question based on this situation.
Please do not forget to generate a question.
Answers
The ideal gas law, which relates the pressure, volume, temperature, and number of moles of an ideal gas, can be applied to real-world situations. By considering a specific scenario and applying the ideal gas law, we can analyze the behavior of gases and make predictions about their properties.
Let's consider a situation where a scuba diver is exploring underwater at a depth of 30 meters. We can apply the ideal gas law, specifically the form known as Boyle's law, which states that the pressure and volume of a gas are inversely proportional at constant temperature.
Question: How does the pressure of the gas in the scuba tank change as the diver descends to a depth of 30 meters, assuming the temperature remains constant?
To answer this question, we can use the ideal gas law equation PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature. By keeping the temperature constant, we can observe the relationship between pressure and volume as the diver descends and calculate the change in pressure based on the change in volume.
To learn more about Boyle's law click here:
brainly.com/question/30367133
#SPJ11
A sample of the greenhouse gas, methane ( CH4 ) at a pressure of 121.59 kPa, a volume of 31 liters, and a temperature of 360.15 K. How many moles (n) of gas and grams (g) of gas are in the sample
Answers
PV = nRT
where:
P = pressure (in Pa)
V = volume (in m^3)
n = number of moles
R = universal gas constant (8.31 J/(mol·K))
T = temperature (in K)
First, we need to convert the given pressure, volume, and temperature to SI units:
Pressure = 121.59 kPa = 121.59 x 10^3 Pa
Volume = 31 L = 31 x 10^-3 m^3
Temperature = 360.15 K
Now we can plug in the values and solve for n:
n = (PV) / (RT)
n = (121.59 x 10^3 Pa * 31 x 10^-3 m^3) / (8.31 J/(mol·K) * 360.15 K)
n = 1.48 mol
So the sample contains 1.48 moles of methane.
To find the mass of the gas, we can use the molar mass of methane:
Molar mass of CH4 = 12.01 g/mol (for carbon) + 4 x 1.01 g/mol (for hydrogen) = 16.05 g/mol
Now we can use the formula:
mass = n x M
mass = 1.48 mol x 16.05 g/mol
mass = 23.74 g
So the sample contains 1.48 moles and 23.74 grams of methane.
Determine the isotope symbol that fits each description. a. 68 neutrons, 47 electrons b. mass number = 197, 79 electrons c. atomic number = 86, 136 neutrons d. atomic number = 76, mass number = 192
Answers
The isotope symbol that fits each description is as follows: 68 neutrons, 47 electrons is Ag b. mass number = 197, 79 electrons is Au c. atomic number = 86, 136 neutrons is Rn d. atomic number = 76, mass number = 192 is Os.
Isotopes are two or more atom types that share the same atomic number (number of protons in their nuclei), location in the periodic table, and chemical element but have distinct nucleon numbers (mass numbers) as a result of having a different number of neutrons in their nuclei. Although the chemical properties of each isotope of a given element are nearly identical, they differ in their atomic weights and physical characteristics.
The name "isotope" refers to the fact that different isotopes of the same element occupy the same location on the periodic table. The word "isotope" is derived from Greek origins that mean "the same place".
to know more about isotope visit
https://brainly.com/question/11680817
#SPJ4
What is the effect of increasing pressure on the equilibrium? N2 + 3H2 ⇔ 2NH3 a) Equilibrium shifts in forward direction. b) Equilibrium shifts in backward direction. c) No effect d) It does not depends on pressure.
Answers
Answer:
a) Equilibrium shifts in forward direction.
Explanation:
If pressure is increased, equilibrium shifts to the side with the fewer moles of gas.
There are 4 moles of gas in the reactants and 2 moles of gas in the products.
The equilibrium will shift in the forward direction towards the products.
Hope that helps.
Answer:
A
Explanation:
Which of the following statements does not describe the structure of an atom? (3 points) a Inside the nucleus of an atom are protons and neutrons. b Protons are positively charged sub-atomic particles. c Electrons are negatively charged sub-atomic particles. d Most of the mass of an atom comes from the electron cloud.
Answers
Answer:
D.
Explanation:
The electron cloud has negligible mass. Most mass come from the nucleus.
Below is an image of a well. Explain what type of well this is and how it works.

Answers
Answer:
This is a artesian well they are able to bring up water with out requiring a pump. This happens when there is enough pressure in the aquifer to push the water up to the surface.
Explanation:
I do not quite understand this, anyway someone can help? I'll give brainliest to the correct answer!

Answers
To solve for number one we must follow these steps:
m = 703.55 g − 345.8 g
m = 357.75 g
Then we solve for the density-
d = 357.75 g / 225 mL
d = 1.59 g/mL
Answer for number 5) 1.59 g/mL
Density= Mass/Volume
therefor- Mass = 65.14g and Volume = 35.4mL
Density = 65.14g/35.4 mL = 1.84 g/mL
Answer for number 6) 1.84 g/mL
Question 7-
g = (0.8765 g/mL)(250.0 mL) = 219.1 g
Answer: 219.1 g
Question 8-
Density = mass/volume
Mass = 1587g
4.5cm x 5.2cm x 6cm = 140.4 cubic-cm
Volume = 140.4 cubic-cm
Mass/volume = 1587/140.4 = 11.3 g/cm^3
Answer: 11.3 g/cm^3
Question 9:
First calculate the volume of iron
49.10mL − 45.50mL = 3.60mL
Density = mass/volume;
density = 28.5g/3.60 mL= 7.92 g/mL
Answer: 7.92 g/mL
Question 10:
Volume = 2500.0g / 10.5g/cm3 = 238.095cm^3
Now we round up the answer to 238cm^3
Answer: 238cm^3
I hope this helped!
What are the three mistakes in the set up below

Answers
In the diagram, we see a distillation process. This is a separation process that takes advantage of differences in the boiling point of a mixture of liquids, so the more volatile liquid will evaporate and be recovered as distillate.
1) The first error seen is that the liquid solution mixture is not heated. In order to separate the liquids, we must heat the mixture by adding heat, and this heat is represented in the diagram with a flame.
2) The second mistake is the position of the thermometer, the thermometer must not be in contact with the liquid, it must be at the height of the bulb so that it is in contact with the vapor.
3) The third error is the exit of the coolant water. The water exchanges heat with the steam, so the steam cools and condenses and the water absorbs the heat, and its temperature increases, so it does not come out cold.
In the following figure we can see the mistakes:

Consider the nuclear equation below. 239/94 Pu—-> X+ 4/2 He. What is X?
Answers
Answer:
X = U (Uranium)
Explanation:
Pu-->235/92 U + 4/2 He
The element X which is involved in the given nuclear reaction is ²³⁵₉₂U.
What is radio active decay?Radio active decay is a process in which an unstable nuclei will emit some particles from the nuclei in the form of energy to get stability.
In the given chemical reaction, parent nuclei (²³⁹₉₄Pu) will emit the alpha particle (⁴₂He) which has a atomic number 2 and atomic mass of 4 as a result of which we get a daughter nuclei Uranium which will get the atomic number 92 and atomic mass of 235.
²³⁹₉₄Pu → ⁴₂He + ²³⁵₉₂U
Hence the element X is uranium (²³⁵₉₂U).
To know more about radioactive decay, visit the below link:
https://brainly.com/question/11117468
#SPJ2
How many moles are in 15.0 g Aluminum
Answers
The number of moles in 15.0 g of Aluminum having an atomic mass of 27 g/mol is 0.556 mol.
Now,
we know that,
number of moles = given mass/molar mass
And, the molar mass of Aluminum is 27 units.
so,
number of moles = 15.0 / 27
= 0.556
hence , 0.556 moles are in 15.0 g of Aluminum.
Learn more about Moles at :
https://brainly.com/question/1427604
PPLLLSSSSSSS HELLLPPP!!!!! What is runoff?
Answers
Answer:
runoff is he draining away of water (or substances carried in it) from the surface of an area of land, a building or structure, etc.
Explanation:
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11