Answers
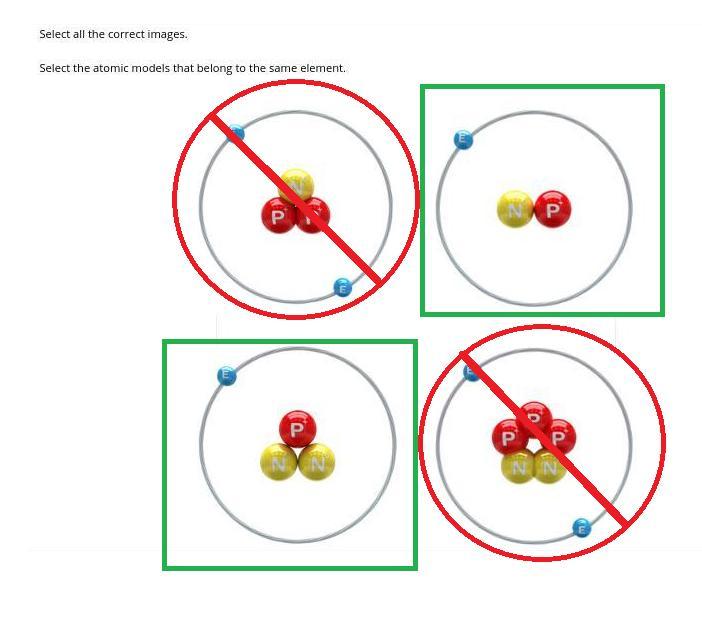
Answer: The atoms in the green boxes are the same element while the others in the red circles are of different elements.
Explanation:
Atoms of the same element MUST have the same number of protons. The number of neutrons may vary in the case of ISOTOPES (in which case the green labelled atoms are isotopes). Both red labelled atoms have a different number of protons from the other two.
Related Questions
With carbon dioxide, what phase change takes place when pressure increases from 1 atm to 10 atm at -40C?
Answers
Answer:
See explanation
Explanation:
The phase changes of carbon dioxide is quite interesting. It is even more interesting that its phase change from gas to solid is dependent on pressure and temperature.
The triple point of CO2 is -56°C at 5.11 atm pressure. This implies that below 5.11 atm, liquid CO2 can not exist. In fact, at -78°C and 1 atm, CO2 is a solid.
Hence, when pressure increases from 1 atm to 10 atm at -40C, CO2 changes from liquid to gas.
5. How long would it take for 2000 coulombs of charge to flow through a motor if the current is 2.5A?
Answers
\(\qquad \qquad\huge \underline{\boxed{\sf Answer}}\)
As we know,
\( \sf \:currrent = \dfrac{charge}{time} \)
where,
Charge is in Coulombs Time is in seconds Current is in AmperesLet's use the given formula ~
\(\qquad \sf \dashrightarrow \: 2.5 = \dfrac{2000}{t} \)
\(\qquad \sf \dashrightarrow \: t= \dfrac{2000}{2.5} \)
\(\qquad \sf \dashrightarrow \: t = \dfrac{2000 \times 2}{2.5 \times 2} \)
\(\qquad \sf \dashrightarrow \: t = \dfrac{4000 }{5 } \)
\(\qquad \sf \dashrightarrow \: t = 800\)
So, the Total time taken by the Charge to flow through a motor is 800 seconds
Or it can be converted into minutes as :
\(\qquad \sf \dashrightarrow \: \dfrac{40}{3} \: \: or \: \: 13.33 \: \: min\)
8. In
studies, scientists do not interfere with what is going on.
Answers
Answer:
Observational
Explanation:
This is the answer because researchers or scientist observe the effect of a diagnostic test, treatment or other intervention without trying to change anything.
What does the hydrosphere include?
Glaciers
Groundwater
Lava
Polar Ice
Answers
The hydrosphere include glaciers, groundwater, and polar ice. That is option A, B and C.
What is hydrosphere?Hydrosphere is defined as the part of the earth that is made up of water which includes the surface of the planet, underground, and in the air.
The water found in the hydrosphere of earth moves in cycles in such a way that non is lost.
The components of the hydrosphere include the following:
oceans, Polar ice,freshwater,surface water, groundwater, glacial water, and atmospheric water vapour.The polar ice is part of the hydrosphere because it is the frozen part of the earth's hydrosphere.
The lava is not part of the hydrosphere but part of lithosphere and it's released when volcanic eruptions occurs.
Therefore is can be concluded that glaciers, groundwater, and polar ice are parts of the hydrosphere while the lava is not part of the hydrosphere.
Learn more about hydrosphere here:
https://brainly.com/question/25796102
#SPJ1
What are the names of the varibles that a scientist uses when performing a scientif investigation
experiment)?
Answers
Answer:
Manipulated (independent) Variable and Responding (Dependent) Variable
PLEASE HELP ME DUDE MY CLASS ENDS IN LIKE HALF A DAY

Answers
Answer:
c.all atoms have the same arrangement for electrons
Answer:
C because the number of protons and neutrons add up to make the number of electrons.
Any 1 know These Chemicals??
Answers
Answer:
A) 1 & 2 only
Explanation:
Seeing that the chemicals present are in question one I take it you are asking about that question.
Decreasing the concentration causes a decrease in reaction process and also the total amount of product you would get out of the given reaction
Decreasing the temperature causes the molecules in this reaction to become less excited or reactive, thus causing the reaction to slow down
Se ard 25g de magneziu de puritate 90%. Ce volum de oxigen se consumă și câti moli dioxid de magneziu se formează
Answers
Answer:
10.5 dm3 O2
0.94 moles de MgO
Explanation:
La pregunta dice explícitamente que el magnesio es puro en un 90%.
Por lo tanto, masa de magnesio puro = 90/100 * 25G = 22,5 g
Número de moles de Mg = 22,5g / 24g/mol = 0,94 moles de Mg
La ecuación de reacción es;
2Mg (s) + O2 (g) ------> 2MgO (s)
Si 2 moles de Mg reaccionan con 1 moles de O2
0.94 moles de Mg reacciona con 0.94 * 1/2 = 0.47 moles de O2
Si 1 mol de O2 ocupa 22,4 dm3
0.47 moles de O2 ocupan 0.47 * 22.4 / 1 = 10.5 dm3
También;
2 moles de Mg producen 2 moles de MgO
Entonces, 0.94 moles de Mg producen 0.94 moles de MgO
Is this correct ? I need help

Answers
Answer:
Yup, I believe so.
Explanation:
Complete the paragraph to describe binary systems.
A binary star system is made of
stars, one of which is brighter than the other. Astronomers are able to detect the dimmer star because its gravity causes the bright star to
. Astronomers can also spot the dimmer star by observing a phenomenon called a(n)
binary. This happens when the dim star passes in front of the bright star.
Answers
Answer: Two, Wobble, Eclipsing.
Explanation:
A binary star system is made of two stars, one of which is brighter than the other. Astronomers are able to detect the dimmer star because its gravity causes the bright star to wobble. Astronomers can also spot the dimmer star by observing a phenomenon called an eclipsing binary.
Answer:
Two, Wobble, Eclipsing.
Explanation:
A buildup of charges in an object is called
Answers
Answer:
Static Electricity
Explanation:
Answer:
static electricity
Explanation:
A neutral atom has 12 protons. In chemical bonding, which is the most likely charge of the ion?
A. 2+
B. 1+
C. 2-
D. 6-
Answers
The recommended daily allowance (RDA) of calcium is 1.0 g for men and women from 19-50 years of age. Calcium carbonate CaCO3 contains 12.0% calcium by mass. How many grams of calcium carbonate are needed to provide the RDA of calcium?
Answers
The 8.33 grams of calcium carbonate are required to provide the RDA of calcium.
The RDA (Recommended Daily Allowance) of calcium is 1.0 g for men and women from 19-50 years of age. Calcium carbonate CaCO3 contains 12.0% calcium by mass. To determine the number of grams of calcium carbonate required to provide the RDA of calcium, the following steps need to be followed:Step 1: Calculate the mass of calcium that is required to meet the RDA for calcium. 1.0 g is the recommended daily allowance (RDA) for calcium. Therefore, we require 1.0 g of calcium. Step 2: Calculate the mass of calcium carbonate that will yield 1.0 g of calcium. Calcium carbonate contains 12.0% calcium by mass.
This implies that 100 g of calcium carbonate contains 12 g of calcium. Then, (12 g of calcium / 100 g of CaCO3) = (1 g of calcium / x)Where x is the mass of CaCO3 required to obtain 1.0 g of calcium. Therefore, solving for x gives: x = (100 g of CaCO3 × 1 g of calcium) / 12 g of calciumx = 8.33 gThus, 8.33 g of calcium carbonate are needed to provide the RDA of calcium. Answer: 8.33 grams of calcium carbonate are required to provide the RDA of calcium.
To know more about calcium carbonate visit:-
https://brainly.com/question/15383829
#SPJ11
Is air a mixture or pure substance
Answers
Answer:
pure substance
Explanation:
According to the following thermochemical equation, what mass of HF (in g) must react in order to produce 690 kJ of energy
Answers
3.00 ×10² g of HF.
What is thermochemical equation?A Thermochemical Equation is a balanced stoichiometric chemical equation that includes the enthalpy change, ΔH. In variable form, a thermochemical equation would look like this:
A + B → C. ΔH = (±) #
The thermochemical reaction is of two types:
Endothermic Reaction Those thermochemical reactions in which heat is absorbed. Change in enthalpy for this reaction is positive.
Exothermic Reaction Exothermic reactions are the reaction in which the heat or the energy is evolved during the reaction.
SiO2 + 4 HF(g) → SiF4(g) + 2 H20(l) ΔH®rxn=-184 kJ
to learn about thermochemical equation go to - brainly.com/question/2874342
#SPJ4
Helium gas collected at a pressure of 0.0045 atm is put into a flexible balloon measuring 350 mL in volume. What volume, in mL, would the balloon be if the pressure were increased to 0.350 atm?
Answers
Evidence: i looked it up
What mass of water is needed, so that when its temperature increases by 24.7°C, the heat energy is 24,183 J?
Answers
The mass of water needed to increase its temperature by 24.7°C and produce a heat energy of 24,183 J is 230.7 grams.
To calculate the mass of water required to increase its temperature by 24.7°C and produce a heat energy of 24,183 J, we can use the specific heat capacity of water. The specific heat capacity of water is 4.18 J/g°C. This means that it takes 4.18 joules of energy to raise the temperature of 1 gram of water by 1 degree Celsius.
Using this information, we can calculate the mass of water needed as follows:
Heat energy = mass x specific heat capacity x change in temperature
Substituting the values given in the question, we get:
24,183 J = mass x 4.18 J/g°C x 24.7°C
Simplifying this equation, we get:
mass = 24,183 J / (4.18 J/g°C x 24.7°C)
mass = 230.7 g
Therefore, the mass of water needed to increase its temperature by 24.7°C and produce a heat energy of 24,183 J is 230.7 grams.
To know more about Mass visit:
https://brainly.com/question/19694949
#SPJ11
A concentration of 120.9132 g of MgO in 3 L of solution will be what molarity (M)? (The atomic weight are: Magnesium: 24.305 g/mol, Oxygen: 15.9994 g/mol). A.3.0 M MgO B.0,5 M MgO C.2.0 M Mgo D.1.0 M Mgo E.12.0 mol MgO
Answers
The molarity of a solution is calculated by dividing the number of moles of a substance by the volume of the solution.
Here, correct answer will be
To determine the molarity of a 120.9132 g of MgO in 3 L of solution, we need to first convert the grams of MgO to moles. This can be done by dividing the mass of the MgO (120.9132 g) by its molar mass (24.305 g/mol for magnesium and 15.9994 g/mol for oxygen).
This gives us a total of 5.04 moles of MgO. We then divide this number by the volume of the solution (3 L) to get the molarity. Therefore, the molarity of 120.9132 g of MgO in 3 L of solution is 1.0 M MgO.
Know more about molarity here
https://brainly.com/question/2817451#
#SPJ11
Answer question number 16. The question is in the image.

Answers
The exercises refer to hydrocarbons. Depending on the type of bond, it will be the termination of the name of the molecule.
For a single bond: End with the suffix -ane
For double bond: Ends in -ene
For triple bond: Ends in -yne
Let's look at each particular case
a) Heptene
Hept- means 7 carbons, so we have a compound with 7 carbons and one double bond. The formula will be:
\(CH_2=CH-CH_2-CH_2-CH_2-CH_2-CH_3\)c)Hexane
6 carbons with a simple bond
\(CH_3-CH_2-CH_2-CH_2-CH_2-CH_3\)d)Pentane
5 carbons with a simple bond
\(CH_3-CH_2-CH_2-CH_2-CH_3\)e)Propyne
3 carbons with a triple bond
\(CH\equiv C-CH_3\)50 mL of unknown concentration of HBr is titrated with 0.500M KOH. It is found that to complete neutralization, 75mL of KOH was used. What was the original volume of HBr that was titrated ?
Answers
The original volume of HBr that was titrated can be calculated as the ratio of the moles of HBr to its concentration.
To determine the original volume of HBr that was titrated, we can use the concept of stoichiometry and the equation balanced for the neutralization reaction between HBr and KOH.
The balanced equation is:
HBr + KOH → KBr + H₂O
From the balanced equation, we can see that the stoichiometric ratio between HBr and KOH is 1:1. This means that for every mole of HBr, we need an equal number of moles of KOH to complete neutralization.
First, let's determine the moles of KOH used in the titration:
Moles of KOH = 0.500 M × 0.075 L = 0.0375 mol
Since the stoichiometric ratio is 1:1, this also represents the number of moles of HBr that were neutralized.
Now, we can calculate the original volume of HBr using the concentration of the unknown solution:
Moles of HBr = 0.0375 mol
Concentration of HBr = unknown (let's assume it is C mol/L)
Volume of HBr = Moles of HBr / Concentration of HBr = 0.0375 mol / C mol/L
For more such questions on volume
https://brainly.com/question/14197390
#SPJ11
write the ground state electron configuration of a lead atom
Answers
Answer:
electronic configuration of Lead at the ground state is [ Xe ] 6 s 2 4 f 14 5 d 10 6 p 2 .
the natural decay of ddt is a first order process with a half-life of 56 days. what is the rate constant, k, of ddt decomposition?
Answers
A first order process' rate constant, k, can be computed using the formula: k = 0.693/half-life.
What does equation mean in its entirety?A equation is a scientific statement indicating that two sums or values are equal, such as 6 x 4 = 12 x 2. When two or more components must be taken into account collectively in order to comprehend or describe the overall situation, this is known as an equation.
What sort of equation would that be?The meaning of an equation in algebra is a scientific statement that demonstrates the equality of two mathematical expressions. For instance, the equation 3x + 5 = 14 consists of the two equations 3x + 5 and 14, which are separated by the 'equal' sign.
To know more about formula visit:
https://brainly.com/question/30313275
#SPJ4
Given that the density of iron is 11.35 grams per centimeter
cubed, what would be the volume of a 1.1 gram piece of iron?
Answers
Answer:
Volume = 0.097 cm³Explanation:
Density of a substance can be found by using the formula
\(Density(\rho) = \frac{mass}{volume} \)From the question
Density = 11.35 g/cm³
mass of iron = 1.1 g
To find the volume substitute the values into the above formula and solve for the volume
That's
Making volume the subject we have
\(volume = \frac{mass}{Density} \)So we have
\(volume = \frac{1.1}{11.35} \)= 0.096916
We have the final answer as
Volume = 0.097 cm³Hope this helps you
Select all the attractive forces associated with solid NaCl salt. Ion-dipole H-bonding London Disperson Dipole-dipole lonic bonding
Answers
The attractive forces associated with the solid NaCl salt is the Dipole-dipole force and the ionic bonding.
The NaCl is the sodium chloride , the NaCl is formed by the ionic bond and called as the ionic compound. The ionic bond is the attractive force that is formed in between the positively charged atom and the negatively charge atom. The ionic bond is formed between the atom by the complete transfer of the electrons.
The NaCl compound is the polar in nature because of the difference in the electronegativity between the sodium and the chlorine atoms, create a permanent dipole. Therefore, the dipole dipole interaction is present in the sodium chloride.
To learn more about attractive force here
https://brainly.com/question/30542287
#SPJ4
How many atoms of barium are in
274.66 g of Ba?
Answers
Answer:
Avogadro's number, 6.022×1023 6.022 × 10 23 , gives us the amount of substances or particles contained in one mole. For example, there are 6.022×1023 6.022 × 10 23 atoms in one mole of Ba, the symbol for the element barium.
To find the number of atoms in 274.66 g of barium, you need to know the atomic weight of barium and convert the weight to moles. The atomic weight of barium is 137.327 g/mol. Using the formula:
mol = weight/atomic weight
we find that 274.66 g of barium is equivalent to 2.00 mol of barium. Since there are Avogadro's number of atoms per mole, the number of atoms of barium in 274.66 g is equal to 2.00 mol * 6.022 x 10^23 atoms/mol = 1.20 x 10^24 atoms.
adipic+acid+contains+49.32%+c,+43.84%+o,+and+6.85%+h+by+mass.+what+is+the+empirical+formula?
Answers
The ratio of atoms determines the empirical formula of adipic acid. As a result, C₂H₂O is the empirical formula for adipic acid.
To determine the empirical formula of adipic acid based on the given percentages of carbon (C), oxygen (O), and hydrogen (H) by mass, we need to convert these percentages into moles and then find the simplest whole number ratio.
Assume we have 100 grams of adipic acid. This assumption allows us to directly convert the percentages into grams.
Mass of C = 49.32 g
Mass of O = 43.84 g
Mass of H = 6.85 g
Convert the masses of each element into moles by dividing by their respective molar masses:
Molar mass of C = 12.01 g/mol
Molar mass of O = 16.00 g/mol
Molar mass of H = 1.01 g/mol
\(\text{Moles of C} = \frac{{49.32 \, \text{g}}}{{12.01 \, \text{g/mol}}} \approx 4.107 \, \text{mol}\)
\(\text{Moles of O} = \frac{{43.84 \, \text{g}}}{{16.00 \, \text{g/mol}}} \approx 2.740 \, \text{mol}\)
\(\text{Moles of H} = \frac{{6.85 \, \text{g}}}{{1.01 \, \text{g/mol}}} \approx 6.792 \, \text{mol}\)
Determine the simplest whole number ratio of the moles by dividing each mole value by the smallest mole value:
\(\text{Moles of C} = \frac{{49.32 \, \text{g}}}{{12.01 \, \text{g/mol}}} \approx 4.107 \, \text{mol}\)
\(\text{Moles of O} = \frac{{43.84 \, \text{g}}}{{16.00 \, \text{g/mol}}} \approx 2.740 \, \text{mol}\)
\(\text{Moles of H} = \frac{{6.85 \, \text{g}}}{{1.01 \, \text{g/mol}}} \approx 6.792 \, \text{mol}\)
Round the mole ratios to the nearest whole number:
Moles of C ≈ 2
Moles of O ≈ 1
Moles of H ≈ 2
The empirical formula of adipic acid is determined by the ratio of atoms. Therefore, the empirical formula of adipic acid is C₂H₂O.
Note: The molecular formula of adipic acid may be a multiple of the empirical formula, indicating a higher number of atoms in the actual molecule.
To know more about the empirical formula refer here :
https://brainly.com/question/14044066#
#SPJ11
how many millimeters of a 0.266 M LiNO3 solution are required to make 150 mL of 0.075 M LiNO3 solution
Answers
Total, 42.37 millimeters of the 0.266 M Lithium nitrate solution are required to make 150 mL of the 0.075 M LiNO₃ solution.
To determine the volume of a 0.266 M LiNO₃ solution required to make 150 mL of a 0.075 M LiNO₃ solution, we can use the equation:
C₁V₁ = C₂V₂
Where;
C₁ = initial concentration of the LiNO₃ solution (0.266 M)
V₁ = volume of the LiNO₃ solution to be measured in millimeters (mm)
C₂ = final concentration of the LiNO₃ solution (0.075 M)
V₂ = final volume of the LiNO₃ solution (150 mL)
Rearranging the equation, we have;
V₁ = (C₂V₂) / C₁
Substituting the given values;
V₁ = (0.075 M) × (150 mL) / (0.266 M)
Converting mL to mm (1 mL = 1 mm), we get:
V₁ = (0.075 M) × (150 mm) / (0.266 M)
Calculating the result;
V₁ ≈ 42.37 mm
Therefore, approximately 42.37 millimeters of the 0.266 M LiNO₃ solution are required to make 150 mL of the 0.075 M LiNO₃ solution.
To know more about Lithium nitrate here
https://brainly.com/question/26319767
#SPJ4
6 spills and 100 squares
If each spill represents one year, what is the half-life of the square?
Answers
Answer:
hi
Explanation:
What is the pH and final composition of the resulting solution if it contains 10-2 M of both NH4Cl and NaHS
Answers
The final composition of the resulting solution of 10^-2 M NH4Cl and NaHS will have a pH of 9.36.
We are given the concentration of NH4Cl and NaHS as 10^-2 M.The ammonium ion (NH4+) will undergo hydrolysis in water and form NH3 and H+.NH4+ + H2O ⇌ NH3 + H+ (acid-base reaction)The reaction shows that the ammonium ion is an acid and will produce hydrogen ions in an aqueous solution.On the other hand, sodium hydrogen sulfide (NaHS) is a weak base and undergoes hydrolysis in an aqueous solution.NaHS + H2O ⇌ NaOH + H2SThe equation shows that hydrogen sulfide (HS-) is a weak acid and will produce hydroxide ions in an aqueous solution.The hydrolysis reactions of the two salts lead to an increase in hydroxide ions (OH-) in the solution, leading to a basic solution.
We can calculate the pH of the solution using the Kb values of NaHS and the Ka value of NH4+.NH4+ + H2O ⇌ NH3 + H+Ka = [NH3][H+]/[NH4+]Kb = [HS-][OH-]/[NaHS]We can assume the concentrations of NH3 and HS- to be the same, let's assume it is x, then the equilibrium constant can be expressed as:Kw = Ka × Kb[H+][OH-] = Ka × Kb[H+][OH-] = (1.8 × 10^-5) × (1.2 × 10^-13) = 2.16 × 10^-18pH + pOH = 14pH + pOH = 14pH = 14 - pOHpOH = -log[OH-] = -log(1.47 × 10^-8) = 7.83pH = 14 - 7.83 = 6.17We can conclude that the pH of the resulting solution will be 9.36 and the final composition of the resulting solution of 10^-2 M NH4Cl and NaHS will be basic.
To know more about solution visit:
https://brainly.com/question/15757469
#SPJ11
2. If a gas at 500 mL has a temperature of 45°C, then what is the new volume when the temperature is increased to 65°C? Show your work.
Answers
Answer:
V2= 531.4 ml
Explanation:
T1= 273+45 °C= 318 Kelvin
T2= 273+65 °C= 338 Kelvin
V1= 500ml *( 0.001L/1ml)= 0.5 Liters
V2= \(\frac{V1 * T2}{T1}\)= \(\frac{0.5 L * 338K}{318K}\) = 0.5314465409 Liters* (1 ml/0.001 L)= 531.4 ml
