Answers
Answer:
position
Explanation:
The position of an object is its location in a space.
Related Questions
(04.02 LC) Based on the cell theory, which of the following is true?
A: Large organisms have fewer cells than small organisms.
B: Small organisms have smaller cells than large organisms.
C: All living things are made of more than one cell.
D: Cells vary in size depending on their type.
Answers
Answer:
c
Explanation:
there are billions of cells in living things. We are made out of millions of cells
Answer:
D is the answer
Explanation:
An isomer of C3H7O undergoes one step oxidation reaction. Answer the following questions due to this reaction.
a) Write a full symbol equation for this reaction.b) Name the proper reagent and catalyst for this reaction.c) Why do you think there is no need to remove the product from the reaction vessel?
Answers
Answer:
C3H7O + O2 → CO2 + H2O
Explanation:
a) The full symbol equation for the oxidation reaction of an isomer of C3H7O can be represented as:
C3H7O + O2 → CO2 + H2O
b) The proper reagent for this oxidation reaction is O2 (oxygen gas). The catalyst required for this reaction depends on the specific conditions. Common catalysts used for oxidation reactions include transition metals such as platinum (Pt), palladium (Pd), or copper (Cu).
c) There is no need to remove the product (CO2 and H2O) from the reaction vessel because they are typically in the gas or liquid phase and do not significantly interfere with the reaction. The product gases can be easily vented out of the vessel, while the liquid water can be left in the reaction mixture. Additionally, the product CO2 is a stable and inert gas, which does not pose any hazards in most cases. Therefore, it is often not necessary to remove the products after the reaction is complete.
5. Use the formula that states the mass-to-volume ratio of a substance to solve the following:
(a) what is the density of a substance that has a mass of 7.9 g and a volume of 4.3 cm³?
(b) what is the mass of a substance that has a density of 8.9 g/cm³ and a volume of 5 cm³?
(c) what is the volume of a substance that has a density of 1.25 g/mL and a mass of 150 g?
Answers
Answer:
Explanation:
Part A
Density equals mass divided by volume. The units for density are g/cm³ or g/mL. Note that 1 cm³ = 1 mL.
d = m/v
Substitute the mass and the volume into the equation and then divide the bottom number into the top number.
d = 7.9 g/4.3 cm³ = 1.8 g/cm³
Part B
The density of a substance can be used as a conversion factor to find either the mass of the substance or the volume of the substance.
5 cm³ x 8.9 g/cm³ = 45 g
Note that cm³ units cancel and you are left with grams.
Part C
We will use the density of the substance to find the volume of the substance.
150 g x 1 mL/1.25 g = 120 mL
Note that you can invert density so the units of g cancel and you are left with mL.
What is the [H3O+] in 0.550 molar C6H5COOH?
Given: pKa = 4.19
A. 5.96 × 10-3 molar
B. 9.38 × 10-3 molar
C. 0.76 × 10-3 molar
D. 5.96 × 103 molar
Answers
Answer:
A. 5.96 × 10^-3 molar
Explanation:
PLATO
How much heat is required to raise the temperature of 67.0g of water from 25.7°C to
66.0°C? The specific heat of H₂O is 4.184J/g°C)
a) 40.3 kJ
b) 11.3 kJ
c) 67.0 kJ
d) 280.3 kJ
e) 2.70 kJ
Answers
The amount of heat required to raise the temperature of 67 g of water from 25.7°C to 66°C is approximately equal to 40.3 kJ. Thus, the answer is option a) 40.3 kJ.
How do you calculate the amount of heat required to raise the temperature of the water to 66.0°C?The amount of heat required to raise the temperature of a substance is given by the equation:
q = m * c * ΔT
Here, q is the amount of heat required to raise the temperature , m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature due to the heat supplied.
Substituting the given values in the equation, we get:
q = (67 g) * (4.184 J/g°C) * (66°C - 25.7°C)
q = 40,332 J
Converting this value to kilojoules we obtain:
q = 40.332 kJ = 40.3 kJ
Thus 40.3 kJ of heat energy is required to raise the temperature of 67g of water from 25.7 °C to 66° C.
Learn more about specific heat capacity here:
https://brainly.com/question/29766819
#SPJ1
Ca and F what are the name of the ionic compounds
Answers
Answer:
Calcium flouride?
Explanation:
Quite self explanatory
Consider the reaction: CO (g) + 2 H2 (g) ⇌ CH3OH (g) where the Kp is 2.26 x 10^4 at 25°C. Calculate ΔGrxn for the reaction at 25°C under standard conditions.
Answers
The standard Gibbs free energy change of the reaction at 25°C is -18,262 J/mol.
What is energy?Energy is the ability to do work. It exists in many forms, such as kinetic energy (energy of motion), potential energy (stored energy), thermal energy (heat), chemical energy (energy stored in the bonds of molecules), electrical energy (energy carried by electrons), and nuclear energy (energy produced in the nucleus of an atom).
The reaction given is a reversible reaction and the given Kp value is at 25°C. Therefore, we can use the equation ΔG°rxn = -RT ln Kp to calculate the standard Gibbs free energy change of the reaction.
We know that R = 8.314 J/mol K and T = 298 K (25°C)
Therefore, ΔG°rxn = - 8.314 J/mol K x 298 K x ln (2.26 x 10⁴)
ΔG°rxn = -18,262 J/mol
Therefore, the standard Gibbs free energy change of the reaction at 25°C is -18,262 J/mol.
To learn more about energy
https://brainly.com/question/29339318
#SPJ1
Write the net ionic equation for the precipitation of nickel(II) phosphate from aqueous solution:
Answers
The net ionic equation for the precipitation of nickel(II) phosphate from aqueous solution is
Na3PO4 + NiCl2 ---> Ni3(PO4)2 + NaCl
What is the ionic formula for nickel II phosphate?Nickel(II) phosphate is an inorganic compound with the formula Ni3(PO4)2. It is a mint green magnetic force solid that is insoluble in water. The cause of nickel (II) phosphate is established when the nickel (II) ion from nickel (II) sulfate unites with the phosphate ion from sodium phosphate as shown in the net ionic equation given below.
Solutions of sodium sulfide and nickel (II) sulfate are assorted. Na2S+ NiSO4→ Na2SO4 + NiS Nickel (II) sulfide is the precipitate.
So we can conclude that Solid nickel (II) phosphate is formed, meaning it is called a precipitate.
Learn more about phosphate here: https://brainly.com/question/655017
#SPJ1
Consider the following diagram of a chemical reaction.
A series of four increasingly more complex chemical structures. The first diagram shows that when heat is applied to R single bond O single bond O single bond R, it creates 2 R single bond O with 1 electron dot. The next structure brings in more complex structures and other atoms, such as H and C. This process continues until the final molecule has a long chain of six starting with R O and ending with C with a single electron dot. There are four single side chains, 3 P h and 1 H.
What is the product that is formed in this reaction?
a polymer
a monomer
an alkyl halide
a carboxylic acid
Answers
Answer:
The answer is A
Explanation:
on EDG 2021
Answer:A
Explanation:
HELP,QUIZ!!!
Why does your tile floor feel colder on your feet than carpet?
options
-because the tile is a lower temperature than carpet
-because tile conducts energy faster than carpet
-because tile has a smoother texture than carpet
-because tile conducts energy slower than carpet
Answers
Answer:
because tiles conduct energy faster than carpet
Explanation:
when it is winter tiles are as coled as ice an carpet is barley coled when left a tile out in the sun I would burn like a stove but if you left a carpeted it would be warm
When magma goes down into the lower part of the mantie where it is recycled and may come right back up the ocean ridge is called..

Answers
Samples of two metals of equal mass but with different heat capacities are originally at the same temperature. Il the same amounto1'heat is added to both samples, for which metal will the final temperature be lower (assume that no phase change, such as meltng, occurs).
Answers
The heat capacity corresponds to the energy needed to raise one degree of temperature for one gram of substance. That is, the greater the heat capacity, for the same mass, the greater the energy required to raise the temperature of the material.
Therefore, between the two metals with the same mass, the same initial temperature, and the same heat added, we can say that the one with the higher heat capacity will present a lower final temperature.
______ + _______ --> H2O + FrF Complete and balance the equation representing neutralization reaction.
Answers
The general form of a neutralization reaction is HF + FrOH → FrF + H₂O
Which of the following is the formula for a neutralisation reaction?We refer to this as a neutralisation reaction. Only this reaction, which produces NaCl and water as products, is a neutralisation reaction since it involves HCl and NaOH. The resulting response is listed below: NaCl(aq) + H₂O = HCl(aq) + NaOH(aq) (l)
Which of these reactions neutralises an effect?The interaction of H⁺ ions and OH⁻ ions produces water in a neutralisation reaction, which occurs when an acid and a base combine to make water and a salt. The neutralisation of a strong acid and strong base yields a pH of 7.
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
What does Newton’s first law of motion state?
Answers
Answer:
Newton's first law states that every object will remain at rest or in uniform motion in a straight line unless compelled to change its state by the action of an external force. This is normally taken as the definition of inertia. ... If that velocity is zero, then the object remains at rest.
Explanation:
Answer:
Before Galileo and Newton, many people thought that objects lost speed because they had a built-in natural tendency to do so. But those people weren't taking into account the multiple forces here on Earth - for example, friction, gravity, and air resistance - that cause objects to change their speed. If we could see the motion of an object in deep interstellar space, we would be able to observe the natural tendencies of an object that is free from any external influence. In deep interstellar space we would observe that if an object had a speed, it would continue to move with that speed until there was some force causing a change in its motion. Likewise, if an object were at rest in interstellar space, it would remain at rest until there was a force causing a change in its motion.
Explanation:
Hope it helped you =)
how many moles of HCL are required to prepare 0.80L of a 0.5M HCL solution
Answers
Answer:
\(0.4\text{ mol}\)Explanation:
Here, we want to get the number of moles
Mathematically:
\(Number\text{ of moles = molarity }\times\text{ volume}\)We have that as:
\(Number\text{ of moles = 0.5 }\times\text{ 0.8 = 0.4 mol}\)1. Calculate the pH of a solution of 0.2M acetic acid and 0.35M acetate ion. The pk
of acetic acid is 4.8.
pH = pk + log ([A] : [HA])
A.
5.10
B.
5.04
c.
5.25
D.
6.10
E.
6.00
of which
Answers
Answer:
The correct answer is option C.
Explanation:
The pH of the solution with weak acid and its conjugate base is given by the Henderson-Hasselbalch equation:
\(pH=pK_a+\log[\frac{[A^-]}{[HA]}]\)
Where:
\(pK_a\)= The negative logarithm of the dissociation constant of a weak acid
\([A^-]\)= Concentration of conjugate base of a weak acid
\([HA]\)= Concentration of weak acid
We are given a solution with acetic acid and acetate ion.
\(HAc(aq)\rightleftharpoons H^+(aq)+Ac^-(aq)\)
The concentration of acetic acid in a solution= \([HAc]=0.2M\)
The concentration of acetate ion in a solution = \([Ac^-]=0.35M\)
The pK_A of the acetic acid = \(pK_a=4.8\)
The pH of the solution:
\(pH=4.8+\log[\frac{0.35 M}{0.2M}]=5.04\)
5.04 the pH of a solution of 0.2M acetic acid and 0.35M acetate ion.
Hence, the correct answer is option C.
How many mL of a 0.75 N KOH solution
should be added to a 500 mL flask to make
500 mL of a 0.300 M KOH solution?
Answers
The amount of volume of KOH solution that should be added to make 500mL of a 0.300M solution is 200mL.
How to calculate volume?The volume of a solution given the concentration can be calculated using the following expression;
CaVa = CbVb
Where;
Ca = initial concentrationVa = initial volumeCb = final concentrationVb = final volumeAccording to this question, we are to calculate how many mL of a 0.75 M OH solution that should be added to a 500 mL flask to make 500 mL of a 0.300 M KOH solution.
0.75 × Va = 500 × 0.3
0.75Va = 150
Va = 150/0.75
Va = 200mL
Learn more about volume at: https://brainly.com/question/14710169
#SPJ1
Calculate the number of CO2
molecules ( NCO2
) in 0.0734 mol
of CO2
Answers
Answer:
4.42 x 10^22 molecules
Explanation:
1 mole of CO2 has 6.022 x 10^23 molecules
=> 0.0734 x 6.022 x 10^23 = 4.420148 x 10^22 or 4.42 x 10^22
When aluminum metal reacts with iron(III) oxide to form aluminum oxide and iron metal, 429.6 kJ of heat are given off for each mole of aluminum metal consumed, under constant pressure and standard conditions. What is the correct value for the standard enthalpy of reaction in the thermochemical equation below? 2 Al(s) + Fe2O3(s) → 2 Fe(s) + Al2O3(s)
Answers
The standard enthalpy of reaction in the thermochemical equation:
2 Al(s) + Fe₂O₃ (s) → 2 Fe(s) + Al₂O₃ (s) is 859.3 kJ/mol
What is the enthalpy of a reaction?The enthalpy of a given reaction is the amount of heat given off or absorbed when reactants react to form products in that reaction.
A reaction having negative enthalpy change indicates that heat is given off in the reaction and the reaction is said to be exothermic.
A reaction having positive enthalpy change indicates that heat is absorbed in the reaction and the reaction is said to be endothermic.
The amount of heat given off or the enthalpy change when one mole of aluminum metal completely reacts with iron(III) oxide to form aluminum oxide and iron metal is 429.6 kJ of heat.
The correct value for the standard enthalpy of reaction in the thermochemical equation below is determined as follows:
2 Al(s) + Fe₂O₃ (s) → 2 Fe(s) + Al₂O₃ (s)
According to the equation of the reaction, two moles of aluminum reacts completely with iron(III) oxide to form aluminum oxide and iron metal.
The standard enthalpy of reaction = 429.6 kJ/mol * 2
The standard enthalpy of reaction = 859.3 kJ/mol
Learn more about standard enthalpy at: https://brainly.com/question/14047927
#SPJ1
chloramphenicol belongs to the group of:
1.alkaloids
2.antibiotics
3.vitamins
4.esters
Answers
The names and chemical formulas of four substances are shown in the chart.
Carbonic acid- H2CO3
Nitric acid- HNO3
Phosphoric acid- H3PO4
Sulfuric acid- H2SO4
Which substance listed in the chart is made up of the most atoms?
A. Sulfuric acid
B. Phosphoric acid
C. Nitric acid
D. Carbonic acid
Answers
Among the presented substances, Phosphoric acid - H₃PO₄ is the one made up of most atoms.
What is atomicity?Atomicity refers to the number of atoms of each element in a compound. It is given by the corresponding subscripts.
We have 4 substances. To calculate the number of atoms in each one, we will sum the number of atoms that form them.
Carbonic acid - H₂CO₃H + C + O = 2 + 1 + 3 = 6 atoms
Nitric acid - HNO₃H + N + O = 1 + 1 + 3 = 5 atoms
Phosphoric acid - H₃PO₄H + P + O = 3 + 1 + 4 = 8 atoms
Sulfuric acid - H₂SO₄H + S + O = 2 + 1 + 4 = 7 atoms
Among the presented substances, Phosphoric acid - H₃PO₄ is the one made up of most atoms.
Learn more about atoms here: https://brainly.com/question/13453032
Answer:
B
Explanation:
Phosphoric acid
All alkali metals react with water to produce hydrogen gas and the corresponding alkali metal hydroxide. A typical reaction is that between lithium and water: 2Li(s) + 2H2O(1) 2LiOH(aq) + H2(g) How many grams of Li are needed to produce 9.89 g of H₂ ?
Answers
Answer:
69.23g
Explanation:
Find out how many moles is in 9.89g of H2.
number of moles = mass(g) / molar mass
1 is the molar mass of hydrogen (to the nearest whole)
relative molecular mass of H2: 2*1 = 2
number of moles of H2 = 9.89/2 = 4.945
1 mol of H2 is produced from 2 mol of Li
so
4.945 mol of H2 produces 9.89 mol of Li
mass(g) = number of moles * molar mass
7 is the molar mass of Lithium (to the nearest whole)
mass = 9.89 * 7 = 69.23
69.23 grams of Li are needed to produce 9.89 of H2
Using the data collected from the experiment. Calculate the mass of the dried NaCI residue left in the crucible. Do not include the units( which is grams) MassEmpty crucible and cover 33.076Sodium carbonate 0.575PowderDried residue andcrucible and cover. 33.653
Answers
0.577. According to the process, the only possible solid residue on the crucible is NaCl. Therefor the amount NaCl produce is the difference between the crucible with the sample and the empty crucible 33.076-33.653= 0.577
Who ever does this, get brainiest.

Answers
Answer:
C.
Explanation:
They will need to know the influence of gravitational force on objects because gravity can affect an objects weight.
Which is NOT a property of metals?
Answers
Answer:
Silicon????
Explanation:
I searched your question and it gave me that. I hope it helps. If not sorry!
Hydrogen
Carbon
Nitrogen
oxygen
But silicone is both is a metal but also a non metal so it both that’s is call metalloids
Why is a hydrogen outer shell full with only two electrons, whilst a carbon outer shell needs eight electrons?
Answers
Hydrogen outer shell full with only two electrons, whilst a Carbon outer shell needs eight electrons to have completely filled electronic configuration.
Hydrogen has atomic number of 2 i.e., it has total of 2 electrons. As per the rule maximum of 2 electrons can be filled in first energy shell. In case of Hydrogen both the electrons are present in n = 1 shell, here n is principle quantum number. It has completely filled electronic configuration and also the most stable electronic configuration.
In case of Carbon, it has total of 6 electrons as it has atomic number 6. Out of 6 electrons two are filled in n = 1 shell , now we are left with 4 electrons. These four electrons are present in n = 2 shell. These four electrons are distributed as 2 electrons in 2s and 2 electrons in 2p and still we can fill 4 more electrons in 2p orbital to get completely filled electronic configuration which is also the stable one . So, Carbon outer shell needs eight electrons.
To know more about electronic configuration refer to the link:
https://brainly.com/question/26084288
#SPJ9
Refer to the graph below. In order to have a saturated solution, how many grams of potassium nitrate, KNO3, need to be dissolved in 100 grams of water
at 20°C. Select the closest measurement. A.60 B.100 C.30 D.20
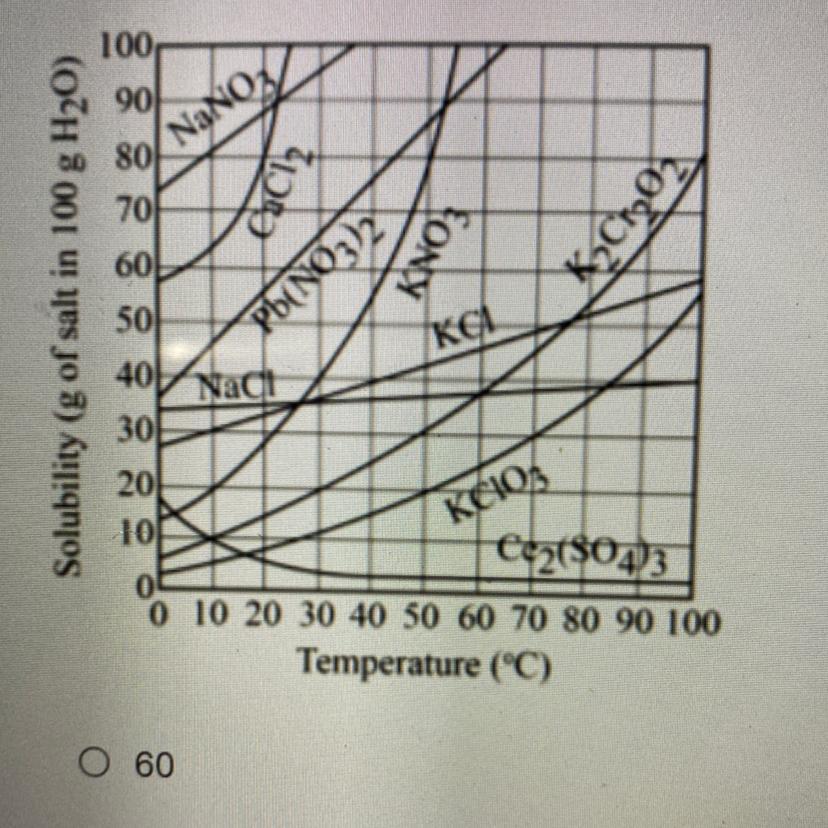
Answers
Answer:
C. 30.
Explanation:
Hello there!
In this case, according to the definition of the solubility, as the maximum amount of a solute that can be completely dissolved in a solvent without the formation of a precipitate, we can see that at the vertical line of 20 °C, on the KNO3 curve, we can see an intercept at about 30.
Such 30 means that just 30 grams of KNO3 will be completely dissolved in 100 g of water at 20°C, by means of its temperature dependent solubility; and therefore the answer is C. 30.
Regards!
It is difficult to break the ionic bonds in a compound because of the
Answers
HELP PLEASE PLEASE PLEASE. Can anyone tell me how to separate the following mixture
A) ethanol in water
B) boiling the mixture of chloride crystals with water
C) pure water from muddy water
D) sodium chloride in water
E) sodium carbonate in water
F) chlorophyll from leaves
G) mixture of acetic acid and alcohol
H) serum from blood sample
I) kerosene from water
J) ammonium chloride in sand
I NEED CORRECT ANSWERS ONLY.
HURRY UP PLEASE. I WILL MARK AS BRAINLIEST
Answers
A) Ethanol in water: Distillation.
B) Boiling the mixture of chloride crystals with water: Evaporation.
C) Pure water from muddy water: Filtration.
D) Sodium chloride in water: Evaporation or Crystallization.
E) Sodium carbonate in water: Filtration or Evaporation.
F) Chlorophyll from leaves: Extraction using a suitable solvent like ethanol.
G) Mixture of acetic acid and alcohol: Distillation.
H) Serum from blood sample: Centrifugation.
I) Kerosene from water: Separatory funnel or Decantation.
J) Ammonium chloride in sand: Sublimation or Dissolving in water and Filtration.
A) Ethanol in water: Distillation can be used to separate ethanol from water based on their different boiling points.
B) Boiling the mixture of chloride crystals with water: By heating the mixture, the water will evaporate, leaving behind the chloride crystals.
C) Pure water from muddy water: Filtration can be used to separate the solid particles (mud) from the water.
D) Sodium chloride in water: Evaporation can be used to separate sodium chloride from water by heating the mixture until the water evaporates, leaving behind the salt.
E) Sodium carbonate in water: Filtration can be used to separate solid sodium carbonate from water, similar to muddy water.
F) Chlorophyll from leaves: Extraction using a suitable solvent like ethanol or acetone can be used to separate chlorophyll from leaves.
G) Mixture of acetic acid and alcohol: Distillation can be used to separate the mixture based on their different boiling points.
H) Serum from blood sample: Centrifugation can be used to separate the serum, which is the liquid part of blood, from the solid components like cells.
I) Kerosene from water: Separatory funnel or decantation can be used to separate the immiscible liquids by pouring off the top layer (kerosene) from the bottom layer (water).
J) Ammonium chloride in sand: Sublimation can be used to separate ammonium chloride by heating the mixture, causing the ammonium chloride to vaporize and then condense back into solid form in a cooler region, leaving the sand behind.
Know more about Sublimation here:
https://brainly.com/question/16789108
#SPJ8
The typical dosage of statin drugs for the treatment of high cholesterol is 10 mg. Assuming a total blood volume of 4.5 L, calculate the concentration of drug in the blood in units of % (w/v)
Answers
Answer:
1.904 ppm
Explanation:
Concentration of drug in units of ppm = mass of solute / (mass of solution ) × 1000000
mass of blood = density of blood × volume = 1.05 g / ml × 5000 ml = 5250 g
mass of solution = mass of blood + mass of solute ( statin) = 5250 + 0.01 g = 5250.01 g
Concentration of drug in units of ppm = (0.01 g / 5250.01 g) × 1000000 = 1.904 ppm
I hope this helps!!
