can aliphatic compounds be detected by a uv detector? what types of compounds can be detected by uv detectors?
Answers
Aliphatic compounds can not be detected by a UV reactor.
Aliphatic compounds are not suitable to be detected by UV reactors. UV or VIS spectroscopy is generally used for determination of quantity in analytical chemistry. The analysis is done for diverse analytes or sample such as transition metal ions, highly conjugated organic compounds and biological macromolecules.
Absorption spectroscopy is complementary with fluorescence spectroscopy. The parameters used in absorption spectroscopy are wavelength, absorbance or transmittance. The only requirement for the absorption spectroscopy is that the sample must be in the UV - Vis region.
Learn more about UV spectroscopy from the link given below.
https://brainly.com/question/16353039
#SPJ4
Related Questions
the globally harmonized system (ghs) of classification and labeling of chemicals is an international approach to hazard communication.
Answers
GHS promotes global understanding and facilitates the safe handling, storage, and transportation of chemicals, protecting human health and the environment.
The Globally Harmonized System (GHS) was developed by the United Nations to address the need for a globally consistent approach to classifying and labeling chemicals. It provides a standardized system for identifying and communicating the hazards associated with chemicals, ensuring that this information is easily understood and recognized across different countries and regions.
GHS uses a set of criteria to classify chemicals based on their intrinsic properties and potential hazards. These hazards include physical, health, and environmental effects. By assigning standardized hazard classes, categories, and pictograms, GHS enables individuals and organizations to quickly recognize and assess the dangers posed by different chemicals.
In addition to classification, GHS also emphasizes the importance of clear labeling and safety data sheets (SDS). Chemical containers must display GHS-compliant labels that include hazard pictograms, signal words, hazard statements, and precautionary statements. These labels help users identify the potential risks associated with a chemical and take appropriate safety measures.
Safety data sheets (SDS), formerly known as material safety data sheets (MSDS), provide detailed information about a chemical's hazards, handling precautions, and emergency response measures. GHS establishes a standardized format for SDS, ensuring that essential information is readily available to workers, emergency responders, and others involved in the handling and transport of chemicals.
The adoption of GHS by different countries and organizations around the world has numerous benefits. It enhances the protection of human health and the environment by promoting consistent hazard communication and facilitating risk management practices. GHS enables improved understanding of chemical hazards, leading to safer handling, storage, and transportation of chemicals. It also supports international trade by providing a common language for hazard communication, reducing barriers and enhancing regulatory compliance.
In conclusion, the Globally Harmonized System (GHS) of classification and labeling of chemicals is an international approach to hazard communication. It standardizes the identification, classification, and labeling of chemical hazards, promoting global understanding and ensuring the safe handling, storage, and transportation of chemicals. By adopting GHS, countries and organizations can enhance safety, protect human health and the environment, and facilitate international trade through consistent and effective communication of chemical hazards.
Learn more about pictograms here:
brainly.com/question/29550697
#SPJ11
Compare these two pictures to the graph above. In which of these situations did they use a catalyst (the left or right)? Why? In which of these two graphs will a reaction actually take place?
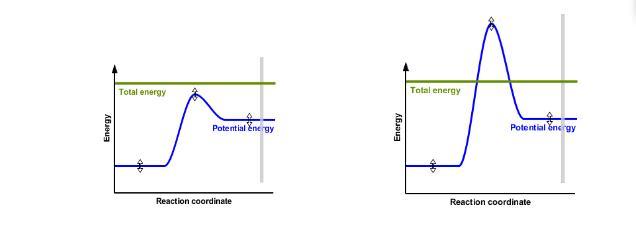
Answers
Answer:
Explanation:
The role of enzymes in a chemical reaction is to : Lower the activation energy while increasing the rate of reaction
The graph shown on the left is an uncatalyzed chemical reaction while the graph on the right is a catalyzed chemical reaction.
Catalyzed chemical reactions have lower activation energy and a faster rate of reaction while uncatalyzed chemical reaction has a higher activation energy and a slower reaction rate. because catalyst increases the rate of a chemical reaction by lowering its activation energy.
Hence we can conclude that The role of enzymes in a chemical reaction is to : Lower the activation energy while increasing the rate of reaction
glycolysis involves the breakdown of one molecule of glucose into
Answers
Glycolysis involves the breakdown of one molecule of glucose into pyruvate and energy. It is the common biological process in all livings to synthesis energy.
What is glycolysis ?Glycolysis is an anaerobic energy source and metabolic pathway that has evolved in nearly all organisms. Although it does not require oxygen, which is why it serves as the first step in anaerobic respiration, it is also the first step in cellular respiration.
The oxidation of glucose molecules, the single most important organic fuel in plants, microbes, and animals, is involved in the process. Glycolysis eventually breaks down glucose into two pyruvate molecules.
Pyruvate enters the citric acid cycle and undergoes oxidative phosphorylation in aerobic conditions, resulting in the net production of 32 ATP molecules.
Therefore, glycolysis involves the breakdown of one molecule of glucose into pyruvate and energy.
Find more on glycolysis:
https://brainly.com/question/27178607
#SPJ1
A chemistry needs a small amount of potassium to carry out an experiment in the lab. She discovered that there is no potassium available. Which of the following elements would be the best available replacement? A. calcium B. magnesium C. sodium D. bromine
Answers
The element that we can be able to use for the experiment in place of potassium is sodium.
What is the best replacement for the potassium?We know that the elements that can be found in the same group does react in the same way. Now we know that we have to look about among the options so that we would be able to know element that is in the same group as potassium.
Given that both sodium and potassium are members of group 1, we have to look out for the element that element thus we have to select sodium.
Learn more about group of elements:https://brainly.com/question/5460947
#SPJ1
VERY URGENTTT
how many moles of h2o is produced when 12 grams of ch4 completely react?
A. 0.75 moles
B. 27 moles
C. 0.67 moles
D. 1.50 moles
Answers
Answer: A
Explanation:

which of the following compounds has a larger lattice energy licl or csbr
Answers
CsBr has a larger lattice energy than LiCl because Cs+ has a larger ionic radius and a greater charge than Li+.
The lattice energy of an ionic compound is determined by the strength of the electrostatic attraction between the ions in the solid crystal lattice. This attraction is influenced by the charges on the ions and the distance between them. The larger the charge on the ions, the greater the lattice energy, and the smaller the distance between them, the greater the lattice energy.
Br- also has a greater charge density than Cl-, making the electrostatic attraction between Cs+ and Br- stronger than that between Li+ and Cl-. Therefore, CsBr has a higher lattice energy than LiCl.
Learn more about lattice energy, here:
https://brainly.com/question/31730061
#SPJ1
a a reaction orrcurs in a calorimeter that contains 2300g of water at 30c. the reaction releases 9.66 *10^3 j of heat. if the specific heat capacity of water is 4.184 j*g*c what is the final temperature of the water
Answers
The final temperature of the water is 33.02°C.
Here's the solution:
Initial temperature of water (T1) = 30°C
Mass of water (m) = 2300g
Specific heat capacity of water (c) = 4.184 J/g°C
Heat released by the reaction (q) = 9.66 * 10^3 J
Final temperature of water (T2) = (T1 + q/mc)
= (30°C + 9.66 * 10^3 J / 2300g * 4.184 J/g°C)
= 33.02°C
The heat released by the reaction is absorbed by the water, causing the temperature of the water to increase.
The final temperature of the water is calculated by adding the heat released by the reaction to the initial temperature of the water and dividing by the mass of the water and the specific heat capacity of water.
Thus, the final temperature of the water is 33.02°C.
To learn more about specific heat capacity :
https://brainly.com/question/29792498
#SPJ11
Propose an efficient synthetic route (using all reagents and materials necessary to perform the following transformations. draw all reagents necessary as well as the product of each step which will become the reactant for the next step.
Answers
When using specialized reagent, the reaction in organic chemistry is carried out by converting one functional group to another functional group.
Cyclohexane carboxaldehyde, which is transformed into 1-cyclohexylethanone, is the suggested starting material. (Aldehyde to ketone conversion. As a result, the reaction takes place in two steps: Aldehyde and Grignard's reagents interact to form alcohol: Secondary alcohol is created when methyl magnesium bromide and cyclohexane carboxaldehyde combine. As a result of the secondary alcohol's reaction with PCC (pyridine chlorochromate), the intended product, ketone, is produced. When using specialized reagents like oxidizing reagents, reducing reagents, Grignard's reagents, the reaction in organic chemistry is carried out by converting one functional group to another functional group.
Learn more about magnesium here-
https://brainly.com/question/1533548
#SPJ4
what conditions would be needed to form needles? group of answer choices temperature of -20 oc and supersaturation of 0.16 g/m3 temperature of -5 oc and supersaturation of 0.18 g/m3 temperature of -2 oc and supersaturation of 0.2 g/m3 temperature of -30 oc and supersaturation of 0.05 g/m3
Answers
To form needles, the conditions would need to include a specific temperature and level of supersaturation. Supersaturation refers to a solution that contains more of a dissolved substance than can be normally accommodated at a specific temperature.
In this case, the supersaturation levels range from 0.05 g/m3 to 0.2 g/m3 and the temperatures range from -30 oC to -2 oC.
However, the specific conditions that would result in needle formation depend on the substance being dissolved. Without knowing the substance in question, it is difficult to determine which combination of temperature and supersaturation would result in needle formation. Different substances have different solubility characteristics and therefore require different conditions to reach supersaturation.
In general, needle-like crystals tend to form in highly supersaturated solutions when the rate of crystal growth is limited by a low diffusion rate. This results in a slow and steady growth of needle-like crystals. In order to determine the specific conditions required for needle formation, a detailed analysis of the solubility characteristics of the substance in question would be necessary.
To know more about Temperature visit:
https://brainly.com/question/5421090
#SPJ11
The following equation illustrates the chemical reaction that occurs in a plant.
Water + carbon dioxide + light energy → sugar + oxygen.
Based on the law of conservation of energy, what happened to the light energy in the reaction?
A) It was produced by the plant.
B) It was destroyed
C) It was changed into oxygen gas
D) It was converted into chemical energy stored in the sugar.
Answers
it was produced by a plant
3. As a ray of white light passes through a prism, dispersion occurs. Which among the color of
the visible spectrum refracted the MOST?
C. Green
A. Yellow
B. Red
D. Violet
Answers
Your answer is option b
i.e. Red
because refractive index of red is more as compared to green, yellow and violet...
pleaseeeeeee heeeeeeeeelp

Answers
Answer:
You plot the points on graph according to the the table
Once you plotted every point on the table, you connect the dots.
Example: Jan (Month) Go Up To 44 , Feb(Month) Go Up 40
I HOPE I HELP AS MUCH AS POSSIBLE
PLZ I NEED HELP I WILL GIVE 100 points for helping me
Silver nitrate reacts with barium chloride. If 39.02 grams of barium chloride are
reacted, how many grams of silver chloride are produced?
Answers
Answer:
4.2 g good luck with the answer
When the heavy isotopes of hydrogen undergo fusion at extremely high temperatures, _______.
Answers
When the heavy isotopes of hydrogen undergo fusion at extremely high temperatures, they release a tremendous amount of energy.This process is known as nuclear fusion.
Nuclear fusion occurs when two light atomic nuclei combine to form a heavier nucleus. In the case of heavy hydrogen isotopes, deuterium (D) and tritium (T), the fusion reaction can be represented as follows:
D + T -> He + n + Energy
In this reaction, deuterium and tritium nuclei fuse together to form a helium nucleus (He) along with the release of a neutron (n) and a tremendous amount of energy.
The high temperatures required for nuclear fusion are necessary to overcome the strong electrostatic repulsion between positively charged atomic nuclei. By providing enough thermal energy, the kinetic motion of the nuclei allows them to approach closely enough for the strong nuclear force to take effect and bind them together.
Learn more about nuclear fusion at https://brainly.com/question/14019172
#SPJ11
Use the oxidation states as indicated by the superscript numbers to answer the questions. k( 1)cl( 5)o3(-2) → k( 1)cl(-1) o2(0) which element is oxidized? which element is reduced? which element does not change in the oxidation state? how many electrons will be moved to have a balanced reaction?
Answers
Oxygen gets oxidized and potassium and chlorine get reduced. The number of electrons moving to have a balanced reaction is 6.
What is an oxidation number?The number of allocated elements in a chemical combination is termed an oxidation number. The oxidation number is generally the count of the number of electrons that a molecule or atom shares, loses, or gains during bond formation.
The given chemical equation will be
\(\rm 2K ^+ + 2Cl^+ + 2O_3^{2-} = 2KClO_3\)
In this reaction, oxygen gets oxidized and potassium and chlorine get reduced.
The number of electrons moving to have a balanced reaction will be
Number of electron = 2 × 3 = 6
More about the oxidation number link is given below.
https://brainly.com/question/10079361
Answer:
Answer down below
Explanation:

Different organisms have different "habitats." These are all examples of habitats: The tops of banana trees in a rainforest The moist area inside a log on a forest floor The sand on the bottom of a shallow area of the ocean Which survival need best defines "habitat"? oxygen or carbon dioxide someplace to live food water
Answers
Answer:
Oxygen/Carbon dioxide
Explanation:
The basic description of living things are organisms which have a cell system to produce energy with either primarily via respiratory exchange abilities, by the existence .
The primal survival need for a possessing a habitat would have to be oxygen/carbon dioxide present,
I hope this was helpful.
Answer:
Habitat
Explanation:
Thats what its called when an animal has a home there habitat
What is the empirical formula of N4O8
Answers
The empirical formula of N₄O₈ is NO₂.
Understanding Empirical FormulaThe empirical formula of a compound gives the simplest whole number ratio of the atoms present in the compound.
To determine the empirical formula of N₄O₈, we need to find the ratio of the number of atoms of each element present in the compound.
One way to do this is to divide the number of atoms of each element by the greatest common factor of all the numbers.
The subscripts of N₄O₈ are already in their lowest possible ratio, so we can directly write the empirical formula as NO₂.
Therefore, the empirical formula of N₄O₈ is NO₂.
Learn more about Empirical Formula here:
https://brainly.com/question/1603500
#SPJ1
construct the molecular orbital diagram for he2+2 .
Answers
The molecular orbital diagram is attached below.
The molecular orbital diagram for He₂²⁺ can be constructed by following the general rules for filling molecular orbitals. He₂²⁺ is formed by removing two electrons from the helium atom (He), resulting in a He²⁺ ion with a 2+ charge.
In the molecular orbital diagram, there are two helium atoms (He) that combine to form a molecular ion. Each helium atom has two electrons, and since two electrons are removed, the total number of electrons in the system is 2.
First, we start by filling the lowest energy molecular orbital with two electrons, following the Pauli exclusion principle and Hund's rule. Since there are only two electrons, both will occupy the lowest energy molecular orbital.
The molecular orbital diagram for He₂²⁺ can be represented as: ↑↓
Here, the upward and downward arrows represent the two electrons in the system. The molecular orbital they occupy is labeled as the bonding molecular orbital (σ). The σ orbital is lower in energy compared to the atomic orbitals of the helium atoms.
Since there are only two electrons in the system, the diagram does not include any additional molecular orbitals.
It's important to note that He₂²⁺ is not a stable molecule. Helium atoms tend to be chemically inert and do not readily form stable compounds or molecules. The molecular orbital diagram presented here is a theoretical representation based on the principles of molecular orbital theory.
To learn more about molecular orbital diagram, here
https://brainly.com/question/30389469
#SPJ4

8.19 Write the electron configuration for each of the following ions, and determine which ones possess noble-gas configurations: (a) Sr*, (b) Ti2, (c) Se2, (d) Ni2, (e) Br, (f) Mn3*.
Answers
The noble-gas configuration possessed by the given ions includes Sr2+ and Br--.Electron configuration refers to the distribution of electrons within the energy levels, sublevels, and orbitals of an atom.
(a) Sr2+ - 1s2 2s2 2p6 3s2 3p6 4s0 3d0, possesses a noble-gas configuration as it has the same electron configuration as the noble gas element Kr.
(b) Ti2+ - 1s2 2s2 2p6 3s2 3p6 4s0 3d2, does not possess a noble-gas configuration.
(c) Se2- - 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6, does not possess a noble-gas configuration.
(d) Ni2+ - 1s2 2s2 2p6 3s2 3p6 4s0 3d8, does not possess a noble-gas configuration.
(e) Br- - 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6, possesses a noble-gas configuration as it has the same electron configuration as the noble gas element Kr.
(f) Mn3+ - 1s2 2s2 2p6 3s2 3p6 4s0 3d4, does not possess a noble-gas configuration.
The electron configuration is typically written using a series of numbers and letters. The numbers indicate the principal energy levels (also called shells), while the letters represent the sublevels (also known as orbitals). The sublevels include s, p, d, and f orbitals, each with a different shape and capacity to hold electrons.
To know more about electron, visit:
https://brainly.com/question/12001116
#SPJ11
An isotope with a low value of N/Z will generally decay through A) spontaneous fission. B) gamma decay. C) alpha decay. D) beta decay. E) electron capture.
Answers
Alpha decay. The N/Z ratio, which represents the ratio of neutrons (N) to protons (Z) in an atomic nucleus, is an important factor in determining the stability of an isotope. Isotopes with low values of N/Z have too few neutrons to maintain a stable nuclear configuration.
Alpha decay is a common decay mode for isotopes with low N/Z ratios. In alpha decay, the nucleus emits an alpha particle (a helium nucleus), which reduces the number of protons and neutrons in the nucleus, bringing it closer to a more stable configuration.
Other decay modes, such as beta decay and electron capture, may also occur in isotopes with low N/Z ratios, but these processes are less common than alpha decay. Gamma decay, on the other hand, is not a decay mode in the same sense as alpha decay, beta decay, or electron capture; rather, it is a process in which a nucleus releases excess energy in the form of gamma radiation. In summary, isotopes with low N/Z ratios will generally decay through alpha decay in order to become more stable. While other decay modes may also occur, alpha decay is the most common process for these isotopes.
To know more about neutrons visit :
https://brainly.com/question/31977312
#SPJ11
Write the correct abbreviation for each metric unit.
1) Kilogram __ 4) Milliliter __ 7) Kilometer __ 2) Meter 5) Millimeter __
8) Centimeter __ 3) Gram __ 6) Liter __ 9) Milligram __
Answers
The correct abbreviation for each metric unit is:
Kilogram - kg, Milliliter - ml, Kilometer- Km, Meter- m, Millimeter - mm, Centimeter - cm, Gram - g, Liter - L, and Milligram - mg.
What is the metric system?The metric system can be described as a system of measurement that succeeded the decimalized system based on the meter. Each of the fundamental dimensions can be expressed by a single base unit of measure.
For quantities derived from the base units of the system, units derived from the base units are used such as the square meter being the derived unit for the area, a quantity derived from length.
Metric units can be described as units based on the meter, gram, or second and decimal multiples or sub-multiples of these. The units of the International System of Units (SI). By extension, they involve units of electromagnetism from the CGS units and SI units systems.
Learn more about Metric units, here:
https://brainly.com/question/19483018
#SPJ1
In a titration of a monoprotic weak acid with sodium hydroxide, the ph at the half-equivalence point is 4. 20. What is the experimental ka value of this acid?.
Answers
Ka value of monoprotic weak acid:
The experimental ka value of this acid is 6.3 × \(10^{-5}\)
Half-equivalence point:
The pH of the solution will be equal to the pKa of the weak acid at the half-equivalence point.At the equivalence point, the strong base OH- will neutralize the weak acid in the case of a weak monoprotic acid, which I'll denote as HA.HA + OH⁻ → A⁻ + H₂OThe moles of OH are equivalent to the moles of the acid since the acid is monoprotic:
At half equivalence point, pH = pKₐ
The pKa is determined by the weak acid's Ka acid dissociation constant.
pKₐ = -log(Kₐ)
Kₐ = \(10^{-pKa}\)
At the half equivalence point,
Kₐ = \(10^{-pH}\)
Given,
pH = 4.20. By putting the value;
Kₐ = \(10^{-4.20}\)
= 6.3 × \(10^{-5}\)
Learn more about the monoprotic weak acid here,
https://brainly.com/question/14365346
#SPJ4
Consider the chemical equations shown here. P4(s) 3O2(g) → P4O6(s) ΔH1 = -1,640. 1 kJ P4O10(s) → P4(s) 5O2(g) ΔH2 = 2,940. 1 kJ What is the overall enthalpy of reaction for the equation shown below? Round the answer to the nearest whole number. P4O6(s) 2O2(g) Right arrow. P4O10(s).
Answers
The enthalpy of the reaction P₄O₆(s) + 2O₂(g) → P₄O₁₀(s) calculated from the enthalpies of the reactions P₄(s) + 3O₂(g) → P₄O₆(s) and P₄O₁₀(s) → P₄(s) + 5O₂(g) is -1300 kJ.
What is enthalpy?
Enthalpy is a thermodynamic property of a system. It is the sum of the internal energy added to the product of the pressure and volume of the system. Enthalpy is denoted as H.
We need to find the enthalpy of the following reaction:
P₄O₆(s) + 2O₂(g) → P₄O₁₀(s) ... (1)
And we know the enthalpies of the reactions:
P₄(s) + 3O₂(g) → P₄O₆(s) ΔH₁ = -1640.1 kJ .... (2)
P₄O₁₀(s) → P₄(s) + 5O₂(g) ΔH₂ = 2940.1 kJ .... (3)
To calculate the enthalpy of reaction (1) using the values of enthalpies of reactions (2) and (3), we need to make the following changes for these two reactions:
1. Invert reaction (3):
P₄(s) + 5O₂(g) → P₄O₁₀(s) ΔH₂ = -2940.1 kJ ... (4)
Now we have the P₄O₁₀ on the side of the product as in reaction (1). The inversion changed the sing of enthalpy ΔH₂.
2. Invert reaction (2):
P₄O₆(s) → P₄(s) + 3O₂(g) ΔH₁ = 1640.1 kJ .... (5)
The compound P₄O₆ is now on the side of the reactant as in reaction (1). The inversion changed the sing of enthalpy ΔH₁.
Now, the addition of reactions (4) and (5)
To get the reaction (1) we need to add reactions (4) and (5)
P₄(s) + 5O₂(g) + P₄O₆(s) → P₄O₁₀(s) + P₄(s) + 3O₂(g)
Now, reaction (1) will be
P₄O₆(s) + 2O₂(g) → P₄O₁₀(s)
The enthalpy value of the reaction (1) can be calculated by the sum of the enthalpies of the reactions (4) and (5):
ΔH = ΔH₁ + ΔH₂
ΔH = 1640.1 + (-2940.1)
ΔH = -1300 kJ
Therefore, the enthalpy of the reaction P₄O₆(s) + 2O₂(g) → P₄O₁₀(s) calculated from the enthalpies of the reactions P₄(s) + 3O₂(g) → P₄O₆(s) and P₄O₁₀(s) → P₄(s) + 5O₂(g) is -1300 kJ.
To learn more about enthalpy, click here:
https://brainly.com/question/7827769
Answer:
option B
p4O6(s) + 2O2(g) Right arrow. P4O10(s)
Explanation:
just did it. edge 2022
Sucrose has the molecular formula c12h22o11. if a sucrose sample contains 9.0x10^24 atoms of hydrogen, how many molecules of sucrose are present in the sample?
Answers
Sucrose has the molecular C₁₂H₂₂O₁₁ . if a sucrose sample contains 9.0x10²⁴ atoms of hydrogen, There are 1.4370 x10²⁶ molecules of sucrose are present in the sample.
Sucrose C₁₂H₂₂O₁₁ contain C₁₂H₂₂O₁₁ Hydrogen
First we should how how many percentage of hydrogen in sucrose
The following formula will be used to determine the percentage of elements present in a molecule:
(Mass of Element / Mass of Molecule) / 100 equals the percentage of an element.
Sucrose's molecular weight is.
= (12)12 + (1)22 + (16)11,
where 12 is the mass of an atom of carbon.
Hydrogen's atomic mass is 1, thus.
16 = Oxygen Atomic Mass
mass sucrose = 144 + 22 + 176
mass sucrose = 342 g/mol
% of H = (22 / 342) ×100 = 6.43 %
to calculate how many molecules sucrose are present we can compare percentage of hydrogen to % sucrose
% hydrogen = % sucrose
atom hydrogen molecules of sucrose
6.43 % = 100%
9.0x10²⁴ molecules of sucrose
molecules of sucrose = 1.4370 x10²⁶ molecules
Therefore, There are 1.4370 x10²⁶ molecules of sucrose are present in the sample.
Learn more about number of molecule at https://brainly.com/question/28027501
#SPJ4
HELPPP. What is the molarity (molar concentration) of a 500.0mL that contains 5.60g of KOH? Please show your work, with units, and include a therefore statement.
Answers
Answer:
.2 M
Explanation:
grams/molar mass=ans./volume(L)=molarity
5.6/56=ans./.500=.2 M
- Hope that helps! Please let me know if you need further explanation.
Complete and balance the following redox reaction in basic solution. Be sure to include the proper phases for all species within the reaction. CIO (aq) + CO2(aq) → CIO₂(g) + CO₂(g)
Answers
The balanced redox reaction in basic solution is: \(CIO(aq) + CO_2(aq) + OH^{-(aq)} \rightarrow CIO_2(g) + CO_2(g) + H_2O(l)\)
To balance this redox reaction in basic solution, we first need to identify the oxidation state of each element in the equation. We see that the oxidation state of chlorine changes from +1 to +4, while the oxidation state of carbon changes from +4 to +2.
Next, we balance the equation in acidic solution, as we normally would, and then add OH⁻ to both sides of the equation to neutralize the H⁺ ions and form water molecules. This adds an equal number of H⁺ and OH⁻ ions to both sides of the equation, so the charge balance is maintained.
After balancing the equation in basic solution, we make sure that the number of atoms of each element is the same on both sides, and that the charges are balanced. Finally, we add the phases of each species to complete the equation.
To know more about redox reaction, refer here:
https://brainly.com/question/13293425#
#SPJ11
1C. Stain used to demonstrate:
Calcium
a. Hall
b. Fontana-Masson
c. Prussian blue
d. Schmorl
e. Rhodanine
f. von Kossa
Answers
The stain commonly used to demonstrate calcium in tissues is the von Kossa stain. This stain utilizes silver nitrate to react with calcium ions, resulting in the formation of black or brownish-black deposits.
The von Kossa stain is often used in histology and pathology to identify calcifications in tissues such as bone, cartilage, and soft tissues. It is important to note that the von Kossa stain is not specific for calcium and can also react with other mineral ions such as magnesium and iron. Therefore, it is necessary to perform additional tests to confirm the presence of calcium. The von Kossa stain is a valuable tool in the diagnosis of various diseases such as osteoporosis, atherosclerosis, and calcified tumors. The stain is also useful in research studies to investigate calcium metabolism and its role in physiological and pathological processes.
Learn more about mineral ions here:
https://brainly.com/question/21300362
#SPJ11
One quart of liquid is equal to 0.946 Liters. Four quarts is equal to one gallon. How many Liters are equal to 10 gallons of gasoline?
Answers
Answer:
37.84 Liters
Explanation:
(see picture)

Please help!!!
Write the symbol and ionic equations for the reaction of
Iron oxide with carbon
Answers
Answer:
Iron (III) oxide reacts with carbon monoxide according to the balanced equation: Fe2O3 + 3CO
What is the difference between a homogenous and a heterogeneous mixture? Give 3 examples of each mixture and explain its uses in our daily lives.
Answers
Answer:
By combining two or more substances, a mixture is produced. A homogeneous solution tends to be identical, no matter how you sample it. Homogeneous mixtures are sources of water, saline solution, some alloys, and bitumen. Sand, oil and water, and chicken noodle soup are examples of heterogeneous mixtures.
Explanation: