calculate the ph of a 0.50 m solution of sodium acetate (nach3coo) given that the ka of acetic acid (ch3cooh) is 1.8 x 10-5.
Answers
The pH of the 0.50 M solution of sodium acetate is 4.74.
Calculate the pH of a 0.50 M sodium acetate solution (NaCH3COO).
Given the Ka of acetic acid (CH3COOH) is 1.8 x 10^-5.
Sodium acetate (NaCH3COO) is the salt of a weak acid and a strong base.
Sodium acetate is a compound that can be used to make a buffer solution that has a pH greater than 7.0. To find the pH of the given solution, the following equation should be used.
NaCH3COO + H2O ⇌ CH3COOH + NaOH
This can be stated as the dissociation of NaCH3COO in water, where it produces a mixture of acetic acid (CH3COOH) and NaOH.
The equation for the ionization of acetic acid is given below.CH3COOH + H2O ⇌ H3O+ + CH3COO-The Ka value for acetic acid is given as 1.8 x 10^-5.
Now that the equations are provided, we can begin solving the problem. Let's use the following formula to solve for the pH:
pH = pKa + log (conjugate base/acid).
To begin, let's calculate the pKa of acetic acid: pKa = -log (Ka) = -log (1.8 x 10^-5) = 4.74.
Next, let's determine the concentrations of the acid and its conjugate base. Because we are working with a 0.50 M solution of sodium acetate, the concentration of CH3COO- ions is also 0.50 M. Since this is a weak acid, the concentration of H3O+ ions produced is equal to the concentration of the weak acid, or [H3O+] = [CH3COOH].
Therefore, we can substitute our values into the pH formula: pH = 4.74 + log (0.50/0.50)pH = 4.74 + log (1)pH = 4.74
The pH of the 0.50 M solution of sodium acetate is 4.74.
Learn more about sodium acetate
brainly.com/question/12924347
#SPJ11
Related Questions
Chymotrypsin has an α-helix that contains 2.5 turns. Approximately how many amino acids are involved in this helix?A. 20.25B. 9C. 13.5D. 3.75E. 2.5
Answers
Chymotrypsin has an α-helix that contains 2.5 turns. Approximately 9 amino acids are involved in this helix.
What is chymotrypsin?
The pancreas produces chymotrypsin. Chymotrypsinogen is its precursor. By cleaving peptide bonds in locations Arg15–Ile16, trypsin activates chymotrypsinogen and generates –chymotrypsin. The "oxyanion hole" and the hydrophobic "S1 pocket" are the results of the interaction between the aminic group (-NH3+) of the Ile16 residue and the side chain of Asp194. Additionally, chymotrypsin causes its own activation by cleaving at positions 14, 15, 146, and 148 to create -chymotrypsin, which is both more active and stable than -chymotrypsin. A three-polypeptide molecule with disulfide bonds connecting them makes up the final product.To know more about chymotrypsin, click the link given below:
https://brainly.com/question/13638833
#SPJ1
Which planet is the farthest away from the Sun? Is this always true? Explain
your answer.
Answers
Answer: Pluto
Explanation: Ok, so Pluto is the farthest. It is 3.7 billion miles away from our sun.
briefly describe any differences or similarities for any ph changes observed for di water compared to a buffered solution when hydrochloric acid was added to each.
Answers
The main difference between DI water and a buffered solution is that DI water does not have a buffer system to resist changes in pH, whereas a buffered solution can effectively maintain its pH when an acid or base is added.
When comparing the pH changes observed for DI water and a buffered solution when hydrochloric acid is added to each, there are some differences and similarities.
DI water, also known as deionized water, is essentially pure water with no dissolved ions.
When hydrochloric acid is added to DI water, the pH of the solution will decrease significantly, becoming more acidic. This is because hydrochloric acid is a strong acid and will release a large amount of hydrogen ions (H+) into the solution.
On the other hand, a buffered solution contains a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. The purpose of a buffer is to resist changes in pH when an acid or base is added. When hydrochloric acid is added to a buffered solution, the buffer system will help maintain the pH of the solution by absorbing some of the excess hydrogen ions and preventing a drastic decrease in pH.
In summary, the main difference between DI water and a buffered solution is that DI water does not have a buffer system to resist changes in pH, whereas a buffered solution can effectively maintain its pH when an acid or base is added.
Learn more about buffered solution in the link:
https://brainly.com/question/8676275
#SPJ11
What can you tell about the density of the object placed in the container below? A. The object is denser than both liquids B. The object is less dense than both liquids C. The object is more dense than liquid A but less dense than liquid B D. The object is more dense than liquid B but less dense than liquid A
Answers
If the object sinks, it is denser than the liquid. If the object floats, it is less dense than the liquid.
Density of objectsThe density of an object is the ratio of the mass of the object and its volume. It is a property that measures how tightly packed the molecules of objects are.
Generally, the density of objects is measured relative to the density of water. The density of water is given as 1 g/mL. Thus, objects whose density is more than 1 g/mL are said to the denser than water while those with less than 1 g/mL are said to be less dense than water.
As an established fact, objects whose densities are greater than the density of water will sink when placed in water. Those whose densities are lesser than the density of water will float when placed in water.
In the same, if an object is placed on any other liquid that is not water, it either floats or sinks depending on whether the density of the object is lesser or greater than that of the liquid respectively.
More on density can be found here: https://brainly.com/question/15164682
#SPJ1
what kind of alcohols can be used to prepare aldehydes
Answers
Alcohols can be oxidized to form aldehydes using reagents such as PCC (pyridinium chlorochromate), Dess-Martin periodinane, or chromic acid (H₂CrO₄).
Alcohols can undergo oxidation reactions to produce aldehydes using various reagents. One commonly used reagent is PCC (pyridinium chlorochromate), which selectively oxidizes primary alcohols to aldehydes without further oxidation to carboxylic acids. PCC is a mild and versatile oxidizing agent that is widely employed in organic synthesis.
Another reagent is Dess-Martin periodinane, which is a highly efficient and selective oxidizing agent for the conversion of primary and secondary alcohols to aldehydes and ketones, respectively. It provides a convenient and mild method for the preparation of aldehydes.
Chromic acid (H₂CrO₄) is also used as an oxidizing agent to convert primary alcohols to aldehydes. However, chromic acid is a stronger oxidizing agent compared to PCC and Dess-Martin periodinane and can further oxidize aldehydes to carboxylic acids if reaction conditions are not carefully controlled.
These oxidizing agents provide useful tools for the synthesis of aldehydes from alcohols in organic chemistry.
Learn more about alcohols here
https://brainly.com/question/28448244
#SPJ11
Use the sample data to construct a 90% confidence interval estimate of the percentage of cell phone users who develop cancer of the brain or nervous system. %
Answers
Using this formula, we can calculate the confidence interval once we have the sample data, without the sample data, it is not possible to provide an accurate confidence interval estimate.
To construct a 90% confidence interval estimate of the percentage of cell phone users who develop cancer of the brain or nervous system, we would need the sample data, specifically the number of cell phone users and the number of users who developed cancer. Without the sample data, it is not possible to provide an accurate confidence interval estimate.
However, if we assume that we have the necessary sample data, we can proceed with the calculation. The formula for calculating a confidence interval for a proportion is:
Confidence interval
\(=�^±�×�^(1−�^)�Confidence interval= p^ ±z× np^ (1− p^ ) where:�^p^\)
is the sample proportion (number of users with cancer divided by the total number of cell phone users).
\(�\)
z is the z-score corresponding to the desired confidence level (90% confidence level corresponds to a z-score of approximately 1.645).
\(�\)
n is the sample size (total number of cell phone users).
Using this formula, we can calculate the confidence interval once we have the sample data.
Learn more about confidence from below link
https://brainly.com/question/333719
#SPJ11
which process has the larger entropy change: melting ice or boiling water? which process has the larger entropy change: melting ice or boiling water? melting ice boiling water g
Answers
Boiling water has a larger entropy change compared to melting ice. Entropy is a gauge of a system's unpredictability or disorder. A substance's particles have more flexibility to move when it changes from a solid to a liquid or from a liquid to a gas, which causes an increase in disorder and unpredictability. This rise in entropy often follows the rise in molecular randomness.
When ice melts, the arrangement of its particles changes from one that is more structured and organized in the solid state to one that is more random and disordered in the liquid state. Entropy rises as a result of this.
The arrangement of the particles changes from being very tightly packed in the liquid form of water to being much more dispersed and randomly distributed in the gas state as it boils and turns into steam. Compared to ice melting, this increase in volume and the particles' ability to move about causes a far bigger increase in entropy.
In conclusion, melting ice causes a smaller rise in entropy than boiling water does because gaseous particles are more dispersed and random than liquid ones.
To know more about entropy:
https://brainly.com/question/31114987
#SPJ2
I need help with these two questions

Answers
halogens have 7
allali have 2
noble gases have 8
K = 1
P = -3
Ra= 7
Cl = -1
N = 5
O = 6
you measure out 22.658 g of solid sodium hydroxide on a balance. how many moles of sodium hydroxide do you have?
Answers
You have 0.44 moles of sodium hydroxide.
To calculate this, you first need to find the molar mass of sodium hydroxide. The molar mass of sodium hydroxide is 39.997 g/mol.
Next, divide the mass of sodium hydroxide you have (22.658 g) by the molar mass of sodium hydroxide (39.997 g/mol).
22.658 g / 39.997 g/mol = 0.44 moles of sodium hydroxide.
Know more about moles
https://brainly.com/question/15356425
#SPJ11
What is the volume of a 492.2g sample of Kr gas?
0.007601 L
131.6 L
5.870 L
21.97 L
Answers
Answer:
131.6 L
Explanation:
Ideal gas law is valid only for ideal gas not for vanderwaal gas. Therefore, the correct option is option B that is 131.5l. There is no force of attraction between the particles.
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature. Ideal gas is a hypothetical gas. Vanderwaal behave as ideal gas at high temperature and low pressure.
Mathematically,
PV=nRT
where,
P = pressure=1 atm
V= volume=?
n =number of moles=given mass/ molar mass
= 492.2/ 83.79
= 5.87 moles
T =temperature = 273K
R = Gas constant = 0.0821 L.atm/K.mol
1× V = 5.87 × 0.0821 L.atm/K.mol × 273 K
V =131.5l
131.5l is the volume of a 492.2g sample of Kr gas.
Therefore, the correct option is option B that is 131.5l.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ6
(28) CCC Cause and Effect Young's modulus is a measure
of a solid material's stiffness. A highly ductile
material will typically have a very low modulus. The
table shows several different metals, their crystalline
structure, and their Young's modulus. Describe the
pattern you observe and explain the connection
between structure and stiffness.

Answers
From the table, it is very clear that as the crystallinity increases, its stiffness also increasing. The FCC structures are having less crystallinity and is more ductile than BCC structure.
What is ductility of a material ?Ductility of a material is the ability to make very thin elongated wires. Whereas, stiffness of a material is the resistance to any elongation or compression.
The more crystalline the material, more will be the stiffens. The material with higher Young's modulus (Y) are more crystalline and stiff. As Y decreases, stiffness decreases, the material becomes ductile.
Because the body-centered cubic (BCC) lattice is not tightly packed despite being cubic, the face-centered cubic (FCC) crystalline structure has greater ductility than the BCC.
Due to the fact that a hexagonal close-packed (HCP) lattice is densely packed but not cubic, HCP metals are less ductile than BCC and FCC metals.
Find more on ductility:
https://brainly.com/question/22212347
#SPJ9
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
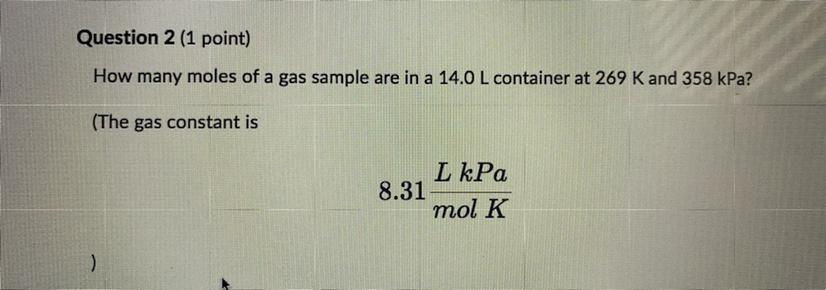
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
a. In a chemical reaction two gases combine to form a solid.What do you expect the sign of ΔS to be?
Answers
Answer:
yes
Explanation:
We're no strangers to love
You know the rules and so do I (do I)
A full commitment's what I'm thinking of
You wouldn't get this from any other guy
An electron in an atom of hydrogen goes from energy level 6 to energy level 2. What is the wavelength of the electromagnetic radiation emitted?
Answers
Answer:
410 nm
Explanation:
when an electron falls from ni=6 to nf=2 , a photon of wavelength 410 nm is emitted.
When an electron falls then the wavelength of the electromagnetic radiation emitted is = 410 nm.
What is electromagnetic wavelength?
When The Electromagnetic wavelength directs to the measured distance between the trough or crest of each adjacent wave generated by the electromagnetic disturbance.
When the wavelength of electromagnetic radiation is comparable to the de Broglie wavelength of its quantum (photon).
Now we are Showing that the wavelength of electromagnetic radiation is comparable to the de Broglie wavelength of its quantum (photon).
Therefore, when an electron falls from ni=6 to nf=2, a photon of wavelength 410 nm is emitted.
Find more information about Electromagnetic wavelength here:
https://brainly.com/question/17212121
what are the three most typical kinds of radiation?
Answers
How many grams of H20 can be produced with 6.3 moles of O2?
Answers
Answer:
For the reaction
2
H
2
+
O
2
→
2
H
2
O
, how many moles of water can be produced from 6.0 mol of oxygen?
Chemistry Mole Ratios
1 Answer
Stefan V.
Dec 5, 2015
12 moles H
2
O
Explanation:
Your tools of choice for stoichiometry problems will always be the mole ratios that exist between the chemical species that take part in the reaction.
As you know, the stoichiometric coefficients attributed to each compound in the balanced chemical equation can be thought of as moles of reactants needed or moles of products formed in the reaction.
In your case, the balanced chemical equation for this synthesis reaction looks like this
2
H
2(g]
+
O
2(g]
→
2
H
2
O
(l]]
Notice that the reaction requires
2
moles of hydrogen gas and
1
mole of oxygen gas to produce
2
moles of water.
This tells you that the reaction produces twice as many moles of water as you have moles of oxygen gas that take part in the reaction.
You know that your reaction uses
6.0
moles of oxygen. Assuming that hydrogen gas is not a limiting reagent, you can say that the reaction will produce
6.0
moles O
2
⋅
2
moles H
2
O
1
moles O
2
=
12 moles H
2
O
If the frequency factor is 1.2*10^13/s , what is the activation barrier?
The rate constant of a reaction at 33 Degrees Celsius was measured to be 5.8×10^-2 /s.
Answers
Therefore the activation barrier of this reaction is EA=84lkJ⋅mol−1, If the frequency factor is 1.2*10^13/s, The rate constant of a reaction at 33 Degrees Celsius was measured to be 5.8×10^-2 /s.
The Arrhenius equation states that
k=A⋅e−EA/R⋅T
Taking logarithm of both sides gives
lnk = lnA−EA/R⋅T
Where, the rate constant of this particular reaction
k=0.055ls−1;
The frequency factor (a temperature-dependent constant A=1.2×1013ls−1as given in the question;
The ideal gas constant R=8.314lJ⋅mol−1⋅K−1;
Absolute temperature (T=32+273.15=305.15lK) at which the reaction take place;
EA the activation barrier (a.k.a. activation energy ) the question is asking for
Solve the second equation for EA:
EA/R⋅T=lnA−lnk
EA=(R⋅T)⋅(lnA−lnk)
=(R⋅T)⋅lnA/k
=8.314lJ⋅mol−1⋅K−1⋅305.15lK⋅ln(1.2×1013s−10/055s−1)
EA=8.4⋅104lJ⋅mo−1
Therefore the activation barrier of this reaction is 84lkJ⋅mol−1
Learn more about activation barrier here:
https://brainly.com/question/11296426
#SPJ4
Which General Hazardous Materials Behavior Model event involves the material being free to travel or disperse, allowing it to move outward from the source?
Answers
The General Hazardous Materials Behavior Model event that involves the material being free to travel or disperse, allowing it to move outward from the source is known as dispersion.
This is the third event in the model and occurs after the release and contact events. During dispersion, the material is no longer contained and can spread through the air, water, or ground.
This can potentially cause harm to people, animals, and the environment. It is important to understand and predict the dispersion of hazardous materials in order to prevent or minimize the impact of an incident.
Learn more about General Hazardous Materials Behavior Model at: https://brainly.com/question/28942478
#SPJ11
What would be the effect of ACh binding to its receptor on the postsynaptic muscle cell?A.) Ca2+ would leak out of the cell as Na+ flowed into the cell.B.) Ca2+ would flow into the cell as Na+ flowed out of the cell.C.) Na+ would flow into the cell and K+ would flow out of the cell.D.) Only Na+ would flow into the cell.
Answers
Na+ would enter the cell while K+ would exit. In the postsynaptic or postjunctional membrane, ACh diffuses and binds to certain receptors.
What happens in the postsynaptic cell when acetylcholine binds to the receptor?In the postsynaptic or postjunctional membrane, ACh diffuses across the synaptic cleft and binds to specific receptors. An altered conformation of a membrane channel that is selectively permeable to both Na+ and K+ results from the binding of ACh to its receptors.
When ACh binds to its receptors, it modifies the structure of a membrane circuit that is selectively permeable to both Na+ and K+. When cholinergic receptors on skeletal muscle fibres interact with each other, ligand-gated sodium channels inside the cell membrane are opened.
The muscle fibre is then exposed to sodium ions, which causes the muscle to contract. By stimulating nonpostsynaptic AChRs, ACh specifically inhibits presynaptic nerve terminal specialisation and postnatal AChR cluster (synaptic differentiation), and by inhibiting postsynaptic AChRs, it inhibits motor short tapered bandwidth or engine axon splitting (synaptic growth).
Ion channels in the muscle fibre membrane are opened by the neurotransmitter acetylcholine (ACh) attaching to postsynaptic receptors.
The complete question is;
What would be the effect of ACh binding to its receptor on the postsynaptic muscle cell?
A.) Ca2+ would leak out of the cell as Na+ flowed into the cell.
B.) Ca2+ would flow into the cell as Na+ flowed out of the cell.
C.) Na+ would flow into the cell and K+ would flow out of the cell.
D.) Only Na+ would flow into the cell.
To learn more about postsynaptic muscle cell refer to:
https://brainly.com/question/29822671
#SPJ4
hlight
How are rocks weathered as in this example seen here?
ame
aining
22:01
Tools
A)
The rocks change their composition.
B)
The rocks are being chemically weathered by the water.
Freezing and thawing of water breaks the rocks into pieces.
D)
Hot lava is pushing on the rock and breaking it into pieces.

Answers
Answer:
B). The rocks are being chemically weathered by the water.
Explanation:
Because the water is slowly destroying the rock
Explain any differences in the pulse rate at rest and after exercise ( in own words btw^^
Answers
Answer:
Pulse rate depends on the activity we do, while exercising theres need of more oxygen so it increases but while at rest body requires less oxygen so low pulse rate.
will magnesium and fluorine atoms most likely form an ionic bond or a covalent bond? 15px
Answers
Magnesium and fluorine atoms will most likely form an ionic bond.
Ionic bonds are formed between elements with a large difference in electronegativity, which is the measure of an atom's ability to attract electrons towards itself. Magnesium and fluorine have a difference in electronegativity of 2.13, which is large enough to form an ionic bond.
In ionic bonds, one atom loses electrons and becomes a positively charged ion (cation), while the other atom gains electrons and becomes a negatively charged ion (anion). In this case, magnesium will lose two electrons to become Mg2+ and fluorine will gain one electron to become F-. These two ions will then attract each other electrostatically to form magnesium fluoride (MgF2), which is an ionic compound.
On the other hand, covalent bonds are formed between elements with a small difference in electronegativity, where atoms share electrons to achieve a stable electron configuration. Magnesium and fluorine have a large electronegativity difference, so they are unlikely to share electrons and form a covalent bond. Therefore, magnesium and fluorine will most likely form an ionic bond.
Learn more about electronegativity here:
https://brainly.com/question/3393418
#SPJ11
What is the name of the region around a magnet where magnetic force acts?
Answers
The name of the region around a magnet where magnetic force acts is the magnetic field.
A magnetic field is a vector field surrounding a magnet, or a current-carrying conductor. It is primarily produced by the flow of charges moving in an undefined motion. It can also produce an external force experienced by other charges placed beside it. This may cause them to move by a force called torque.
The strength of a magnetic field can vary with the strength of the magnet or the conductor, orientation and the amount of electron flowing through the region. Vectors are frequently used to depict magnetic fields, with the magnitude of the vector indicating the force's strength and the direction of the vector indicating the field's direction.
To know more about the magnetic field, refer:
https://brainly.com/question/14411049
#SPJ4
Give the word equation for the reaction between hydrogen and oxygen
Answers
Answer:
Hydrogen gas (H₂) + Oxygen gas (O₂) → Water (H₂O)
Explanation:
Hydrogen reacts with oxygen to form water. The reactants are hydrogen and oxygen and the product is water.
Answer:
Word Equation:
Hydrogen + Oxygen = Water
Hydrogen gas reacts with Oxygen gas to give Water.
The chemical Reaction is as follows:
\(2H_{2} + O_{2}\) => \(2H_{2}O\)
Atmospheric pressure on the peak of Mt, Everest can be as low as 150.0 mmHg, which is why climbers need to bring oxygen tanks for the last part of the climb. If the climbers carry 10.00 L tanks with an internal gas pressure of 3.400 x 10⁴ mmHg, what will be the volume of the gas be when it is released from the tanks?
Answers
Answer:
The volume of the gas when it is released from the tanks will be equal to 10.00 L x (150.0 mmHg / 3.400 x 10⁴ mmHg) = 0.004412 L.
Explanation:
when's things get dropped on the floor what gets on it and like makes it yuk bad and you can't eat no more
Answers
What is a solvent?
A.always water
B.the liquid in the solution
C.the substance in which the solute dissolves
Answers
Answer:
C. the substance in which the solute dissolves
Explanation:
There are two terms in this... the solute and the solvent. The way I learned how to remember this is with a little pirate analogy: The loot (stolen treasure) always goes in the vent. So, the soLUTE goes in the solVENT.
For propanoic acid (HC3H5O2, Ka = 1.3 × 10–5), determine the concentration of all species present, the pH, and the percent dissociation of a 0.100-M solution.
Answers
The percent dissociation of the propanoic acid and the pH of the species are [HCO₃⁻] = 1.53 x 10⁻⁴M and [CO₃²⁻] = 4.8 x 10⁻¹¹ M.
Propionic acid, having the chemical formula CH₃CH₂CO₂H, is a naturally occurring carboxylic acid. It is a liquid with a strong, foul scent that resembles body odour. Propionates or propanoates are names for the propionic acid salts and esters as well as the anion CH₃CH₂CO₂⁻.
Propanoic acid: HC₂H,O₂ (or) C₂H,COOH Concentration of C,H,COOH = 0.290 M ICE table:
C₂H,COOH + H₂O
C₂H,COO+ H₂O*
Acid ionization constant, K 1.3×10= [C₂H,COO-][H₂O*] [C₂H,COOH] x² (0.290-x)
Since C₂H,COOH is a weak acid, we can assume that (0.290x) M= 0.290 M 1.3×10= 0.290 x= √(1.3×10) (0.290) = 1.94×10-3
According to the equilibrium table, [H3O+] = (x)M=1.94×103 M
[C₂H,COO-]=(x)M= 1.94×103 M [C₂H,COOH ]=(0.290-x)M = (0.290-1.94×103) M s 0.28806 M
s 0.288M
pH = -log[H₂O* ] = log(1.94×103) = 2.71 рон = 14.00-pH = 14.00-2.71 = 11.29 [OH-]= 10-10-1129 = 5.13×10-12 M
Percent dissociation = HCHO ] 1.94×10-3 0.290 M ×100% -x100% 0.669% = 0.67%.
Learn more about Propanoic acid:
https://brainly.com/question/20318658
#SPJ4
2. Landmasses and bodies of water affects typhoon. Whichof thesedifferentiate the characteristics of landmasses and bodies of water?
A. Landmasses have more water vapour than bodies of water,
B. Landmasses produce strong wind and heavy rain while bodies of
water cannot.
C. Landmasses strengthen typhoon while bodies of water disrupt
the spin of a typhoon.
D. Landmasses disrupt the spin of a typhoon while bodies of water
strengthen typhoon.
Answers
Answer:
i think
D. Landmasses disrupt the spin of a typhoon while bodies of water
strengthen typhoon.
Compare a mixture and a compound. How are they alike?
Contrast a mixture and a compound. How are they different?
Answers
Answer:
gnzl8303
gnzl8303vvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvv
Explanation:
Answer:
how they are alike: Both compound and mixture are combined in a definite ratio or in any proportion. Both compound and mixture consist of two or more substances/elements. Both compounds and mixtures have physical and chemical properties.
how they are different: The chemical composition of compounds is always fixed. A mixture can have a variable composition of the substances forming it. Mixtures can either be homogeneous or heterogeneous in nature. The constituents of a compound can only be separated by either chemical or electrochemical methods (like extraction).
Explanation: