Answers
Using the molecular weight a calculator or the molar mass of CH₃CH₂OH, 1 gram Ethanol = 0.021706834440237 mole.
Is ethanol used to make gasoline?Ethanol is an renewable transportation fuel that is produced in the United States. Ethanol contributes to lower emissions when used in low-level blends like E10 10% ethanol, 90% fuel), E15 10.5% in 15% ethanol as a or E85 flex boost)—a gasoline-ethanol mix containing 51% to 83% a solution of depending on geography or season.
Why is ethanol classified as an alcoholic beverage?Ethanol (CH₃CH₁OH) is a chemical compound (alcohol) that contains the group hydroxyl (OH) bonded to a the carbon atom. Ethanol is made by fermenting crop residue such as sugar cane, maize, and manioc.
To know more about ethanol visit
brainly.com/question/25002448
#SPJ1
Related Questions
What best describes the location of an atom's electrons?
Answers
Around the Nucleus
How many grams of NaCl

Answers
You would recover 36.525g of NaCl after evaporating all of the water.
How to find the how many grams of NaCl that would be recover when all water is evaporated off of this solution?To find the grams of NaCl that would be recovered after evaporating all the water, we can use the following formula:
mass = moles * molar mass
Where:
Moles = Molarity * Volume
Molarity = 0.250 M
Volume = 2500.0 mL = 2.5 L
Molar mass of NaCl = 58.44 g/mol
mass = 0.250 M * 2.5 L * 58.44 g/mol
mass = 36.525 g
Learn about evaporation here https://brainly.com/question/2013258
#SPJ1
What is a symbol? Write with examples.
Answers
Answer:element symbol
Explanation:
An element symbol is the one or two letter sequence to at identifies an element, typically used through the periodic table
Symbols are abbreviations used in chemistry for chemical elements, functional groups and chemical compounds.
example:
H, Cl ,N , Ne etc.
What kind of reaction is this? C5H8 + 7O2 --> 5CO2 + 4H2O
A. Decomposition
B. Combustion
C. Single replacement
D. Combination
Answers
Answer:
B. Combustion .
Explanation:
Hello.
In this case, since this reaction is started by pentyne and oxygen which are converted to carbon dioxide and water, we can infer this is a combustion reaction because it involves the burnt of a fuel, in this case the pentyne, by using oxygen in order to yield carbon dioxide and water, which are smaller molecules than the original pentyne.
It is important to notice that these reactions are very exothermic because they go to break the C-H bonds in the fuel which release a considerably high amount of energy.
Best regards!
2. Tungsten metal is reacted with carbon to produce tungsten carbide, an extremely hard compound used to make drills and saw blades. How many moles of tungsten carbide will be produced if plenty of tungsten is reacted with 1.25 moles of carbon?
Answers
Answer:
Explanation: Tungsten carbide is formed by the reaction of tungsten (W) and carbon (C) to form tungsten carbide (WC). The balanced equation for the reaction is W + C → WC.
We know that the number of moles of a substance can be calculated using the formula:
moles = mass / molar mass
We also know that the law of conservation of mass states that the total mass of the reactants must equal the total mass of the products. We can use this principle to balance chemical equations.
In this case, we have 1.25 moles of carbon and an unknown number of moles of tungsten. We know that for every 1 mole of carbon, we need 1 mole of tungsten to produce 1 mole of tungsten carbide.
To determine the number of moles of tungsten carbide produced, we can set up the following proportion:
1.25 moles C / 1 mole C = x moles W / 1 mole W
Where x is the number of moles of tungsten needed.
Solving for x, we get x = 1.25 moles W
So, 1.25 moles of tungsten reacts with 1.25 moles of carbon to produce 1.25 moles of tungsten carbide.
What forms of energy are produced when
fossil fuels burn?
Answers
When fossil fuels burn, several forms of energy are produced, including:
Heat energy: The primary form of energy released during fossil fuel combustion is heat. Fossil fuels contain chemical energy stored for millions of years, and when they burn, this energy is released in the form of heat. The heat energy can be harnessed for various purposes, such as heating buildings or generating steam to drive turbines.
Light energy: Burning fossil fuels can also produce light energy in the form of flames or glowing embers. This light energy is a byproduct of combustion.
Mechanical energy: Heat generated by burning fossil fuels can be converted into mechanical energy. This is typically achieved by using heat to produce steam, which drives a turbine connected to a generator. The rotating turbine converts the heat energy into mechanical energy, which is further transformed into electrical energy.
Electrical energy: Through the process described above, burning fossil fuels can ultimately generate electrical energy. The mechanical energy produced by the turbine is converted into electrical energy by the generator. Electrical energy can power various devices, appliances, industries, and infrastructure.
It's critical to note that while burning fossil fuels can produce useful forms of energy, it also results in the release of carbon dioxide and other greenhouse gases. This contributes to climate change and environmental concerns. As a result, there is a global shift towards cleaner and renewable energy sources to mitigate these negative impacts.
You read a primary source and a secondary source that discuss the same
experiment. There is a difference in the conclusions made by these two
sources. Which should you trust more, and why?
A. The primary source, because it is more confusing
B. The secondary source, because it is published in a well-respected
newspaper
C. The secondary source, because it is easier to read
D. The primary source, because it is written by the scientist who did
the work
Answers
Answer:
i think it's D tbh, just cus it was the scientist who did the work
Answer:
It depends on secondary sources and references of that secondary source. If it references just that primary source I would compare both of them and see the difference between them. Generally primary sources are more reliable but this situation is different.
Convert to particles
100 dm3 of Kr**
Answers
100 dm3 of Kr is equal to 100,000,000 cm3 of Kr. Since 1 mole of any gas occupies 22.4dm3 at STP.
What is Kr?Kr is the chemical symbol for krypton, a noble gas element found on the periodic table. It has an atomic number of 36 and an atomic mass of 83.80. Krypton is a colorless, odorless, and tasteless gas that is found in trace amounts in the atmosphere. It is used in a variety of applications, including fluorescent lighting, medical imaging, and welding. Krypton is also used in space exploration, where its inert properties make it a useful gas for deep space probes.
then we can calculate the number of moles of Kr present in the given volume:
Number of moles of Kr = 100,000,000 cm3 / 22.4dm3/mol
= 4,467,849.13 moles of Kr
Since 1 mole of Kr contains 6.022 x 10^23 particles, the number of particles of Kr present in the given volume is:
Number of particles of Kr = 4,467,849.13 moles x 6.022 x 1023 particles/mol
= 2.67 x 1026 particles of Kr
To learn more about Kr
https://brainly.com/question/29078505
#SPJ1
A gas mixture being used to simulate the atmosphere of another planet consists of 320 mg of methane, 175 mg of argon, and 225 mg of nitrogen. Th e partial pressure of nitrogen at 300 K is 15.2 kPa. Calculate (a) the volume and (b) the total pressure of the mixture.
Answers
Answer:
The total pressure is 61.4 kPa, and the volume is 1.31 L.
Explanation:
Based on the given information, a gas mixture comprising 320 mg or 0.320 grams of CH4, 175 mg or 0.175 grams of Ar, and 225 or 0.225 grams of N. The number of moles of the gases presents within the mixture can be determined by using the formula,
Number of moles = Mass/ molecular mass
The molecular mass of methane is 16.04 grams per mole, the molecular mass of Argon is 40 grams per mole, and the molecular mass of Nitrogen is 28.02 grams per mole.
Now, the number of moles of CH4 is,
= 0.320 grams/ 16.04 grams per mole
= 0.0199 moles
The number of moles of Ar is,
= 0.175 grams/40 grams per mole
= 0.0044 moles
The number of moles of N2 is,
= 0.225 grams/28.02 grams per mole
= 0.0080 moles
The partial pressure of nitrogen given is 15.2 kPa or 0.15 atm. Thus, the partial pressure of other two gases will be,
CH4 = (15.2 kPa) (0.0199 moles)/(0.0080 moles)
= 37.8 kPa
Ar = (15.2 kPa) (0.0044 moles)/(0.0080 moles)
= 8.36 kPa
Therefore, the total pressure is 15.2 + 37.8 + 8.36 = 61.4 kPa or 0.606 atm
The total volume can be determined by using the formula,
V = nRT/P
Here n is the total number of moles of the gas, which is 0.0323 moles.
Now putting the values we get,
V = (0.0323 moles) (0.0826 atm*L/mol*K)(300 K)/(0.606 atm)
V = 1.31 L
What dynasty came after China's Five Dynasties and Ten Kingdoms?
Answers
Answer:
The Five Dynasties and Ten Kingdoms (907-960) is a period of great division in Chinese history, and is also the collective name for the Five Dynasties and the Ten Kingdoms.
The Five Dynasties refer to the five regimes located in the Central Plains that were successively replaced after the fall of the Tang Dynasty, namely Later Liang, Later Tang, Later Jin, Later Han and Later Zhou. At the end of the Tang Dynasty, the Five Dynasties and the beginning of the Northern Song Dynasty, there were many separatist regimes outside the Central Plains, among which more than ten separatist regimes such as the former Shu, the latter Shu, and the Northern Han were collectively referred to as the Ten Kingdoms.
Explanation:
Answer:
Mongol Yuan dynasty.
Explanation:
Calculate the amount of Iron-59 remaining after 3 half-lives.
Answers
The amount of Iron-59 remaining after 3 half-lives, given an initial amount of 2.5 grams is 0.3125 grams
How do I determine the amount remaining after 3 half lives?The original amount of a subtance, the number of half lives and the amount of the substance remaining are related according to the following formula:
N = N₀ / 2ⁿ
Where
N is the amount remainingN₀ is the original amount n is the number half livesNow, with the above formula, we shall determine the amount that will remain after 3 half lives. This is shown below:
Original amount (N₀) = 2.5 gramsNumber of half-lives (n) = 3Amount remaining (N) = ?N = N₀ / 2ⁿ
N = 2.5 / 2³
N = 2.5 / 8
N = 0.3125 grams
Thus, from the above calculation, we can conclude that the amount remaining is 0.3125 grams
Learn more about amount remaining:
https://brainly.com/question/28440920
#SPJ1
Complete question:
Given that you originally have 2.5 g of iron-59. Calculate the amount of Iron-59 remaining after 3 half-lives.
How many miles of N2 are in a flask with a volume of 250.0 mL at a pressure of 300.0 kPa and a temperature of 300.0 K?
Answers
hope this helped :)
Which refers to the passing of a wave through an object?
sound
O interference
O transmission
O frequency
O sound
Answers
The term that refers to the passing of a wave through an object is "transmission."
Transmission refers to the process by which a wave passes through an object or medium. In the context of sound, transmission occurs when sound waves travel through different substances, such as air, water, or solids.
When a sound wave encounters an object, it can be transmitted through it, reflected off it, or absorbed by it, depending on the properties of the object and the medium through which the sound is traveling.
For example, when you speak into a microphone, the sound waves produced by your voice travel through the air and are transmitted to the microphone's diaphragm. The diaphragm converts the sound waves into electrical signals, which can then be amplified and reproduced as sound through speakers.
In summary, transmission is the term used to describe the passage of a wave, such as a sound wave, through an object or medium. It is an essential concept in understanding how waves interact with their surroundings and how sound propagates through different materials.
for such more questions on transmission
https://brainly.com/question/18451537
#SPJ8
Chrysanthemic acid occurs as a mixture of esters in flowers of the chrysanthemum (pyrethrum) family.
a. True
b. False
Answers
Answer:
A: True
Explanation:
Statement is true because chrysanthemic acid is formed naturally as a result of production of two molecules of dimethylallyl diphosphate which forms pyrophosphate ester.
Thus, it belongs to the pyrethrum family
A 11.1-g sample of granite initially at 76.0°C is immersed into 22.0 g of water initially at 22.0°C. What is the final temperature of both substances when they reach thermal equilibrium? (For water, Cs=4.18J/g⋅∘C and for granite, Cs=0.790J/g⋅∘C.)
Answers
Answer:
\(T_f=26.7\°C\)
Explanation:
Hello.
In this case, when two substances at different temperature are placed in contact in an isolated container, we can say that the heat lost by the hot substance is gained by the cold substance. In such a way, since granite is at 76.0 °C and water at 22.0 °C we infer granite is hot and water is cold, so we write:
\(Q_{granite}=-Q_{water}\)
In terms of mass, specific heat and change in temperature, we write:
\(m_{granite}C_{granite}(T_f-T_{granite})=-m_{water}C_{water}(T_f-T_{water})\)
Thus, since the temperature is the same for both substance, we can solve for it as shown below:
\(T_f=\frac{m_{granite}C_{granite}T_{granite}+m_{water}C_{water}T_{water}}{m_{granite}C_{granite}+m_{water}C_{water}}\)
By plugging in each variable, we obtain:
\(T_f=\frac{11.1g*0.790\frac{J}{g\°C} *76.0\°C+22.0g*4.18\frac{J}{g\°C} *22.0\°C}{11.1g*0.790\frac{J}{g\°C} +22.0g*4.18\frac{J}{g\°C}}\\\\T_f=26.7\°C\)
Best regards!
Differences between voltage, current and resistance?
Answers
Answer:
Voltage is the measure of electric potential energy per unit charge, current is the flow of electric charge through a circuit, and resistance is the property of a material that opposes the flow of electric current.
Ohm's Law relates these three concepts by stating that current is directly proportional to voltage and inversely proportional to resistance.
Hope this helps!
Assuming a 4.00 litre of a sample gas at 1.00 atm compressed to 0.800 litre at constant temperature. Calculate the final presure of the gas.
Answers
At constant temperature, the final pressure of the compressed gas is 5.0atm.
Given the data in the question;
Initial volume of the gas; \(V_1 = 4L\)Initial Pressure; \(P_1 = 1.0atm\)Final volume; \(V_2 = 0.800L\)Final pressure; \(P_2 = \ ?\)
Boyle's lawBoyle's law states that the volume V of a given quantity of gas is inversely proportional to its pressure P at constant temperature.
It is expressed as;
\(P_1V_1 = P_2V_2\)
Where \(P_1\) is Initial Pressure, \(V_1\) Initial volume, \(P_2\) is Final Pressure and \(V_2\) is Final volume.
To determine the final pressure of the gas, we substitute our given values into the expression above.
\(P_1V_1 = P_2V_2\\\\P_2 = \frac{P_1V_1}{V_2} \\\\P_2 = \frac{1.0atm \ *\ 4L}{0.800L}\\ \\P_2 = \frac{1.0atm \ *\ 4}{0.800}\\\\P_2 = \frac{4.0atm}{0.800}\\\\P_2 = 5.0atm\)
Therefore, at constant temperature, the final pressure of the compressed gas is 5.0atm
Learn more about Boyle's law: brainly.com/question/1437490
can someone help me with my chemistry homework please???

Answers
1.) Lithium and Sulfide:
Formula: \(\bold{Li_{2}S}\)Ion Charges: \(\bold{Li~1+,~Li~1+,~S~2-}\)2.) Lithium and Chlorine:
Formula: \(\bold{2LiCl}\)Ion Charges: \(\bold{Li~1+, Li~1+,Cl~1-,Cl~1-}\)3.) Lithium and Oxygen:
Formula: \(\bold{Li_{2}O}\)Ion Charges: \(\bold{Li~1+,Li~1+,O~2-}\)4.) Lithium and Nitrogen:
Formula: \(\bold{Li_{3}N}\)Ion Charges: \(\bold{Li~1+,Li~1+,Li~1+,N~3-}\)5.) Magnesium and Sulfur:
Formula: \(\bold{MgS}\)Ion Charges: \(\bold{Mg~2+,S~2-}\)6.) Magnesium and Chlorine:
Formula: \(\bold{MgCl_2}\)Ion Charges: \(\bold{Mg~2+,Cl~1-,Cl~1-}\)7.) Magnesium and Oxygen:
Formula: \(\bold{MgO}\)Ion Charges: \(\bold{Mg~2+,O~2-}\)8.) Magnesium and Nitrogen:
Formula: \(\bold{Mg_3N_2}\)Ion Charges: \(\bold{Mg~2+,Mg~2+,Mg~2+,N~3-,N~3-}\)Explanation:______________________________
Lithium and Sulfur: In order to make Lithium Sulfide, There must be 2 Lithium and 1 Sulfur. You transfer the electrons from both Lithium's to the Sulfur.Lithium and Chlorine:In order to make Lithium Chloride, There must be 2 Lithium and 2 Chlorine. You transfer the electrons from both Lithium's to the Chlorines, (One electron for each chlorine.)Lithium and Oxygen:In order to make Lithium Oxide, There must be 2 Lithium and 1 Oxygen. You transfer the electrons from both Lithium to Oxygen. Lithium and Nitrogen:In order to make Lithium Nitride, There must be 3 Lithium and 1 Nitrogen. You transfer the electrons from all 3 Lithium to Nitrogen. Magnesium and Sulfur:In order to make Magnesium Sulfide, There must be 1 Magnesium and 1 Sulfur. You transfer the both electrons from Magnesium to Sulfur. Magnesium and Chlorine:In order to make Magnesium Chloride, There must be 1 Magnesium and 2 Chlorine. You transfer on electron to each Chlorine. Magnesium and Oxygen:In order to make Magnesium Oxide, There must be 1 Magnesium and 1 Oxygen. You transfer both electrons from Magnesium to Oxygen. Magnesium and Nitrogen:In order to make Magnesium Nitride, There must be 3 Magnesium and 2 Nitrogen. You transfer 3 electrons from Magnesium to Nitrogen.______________________________

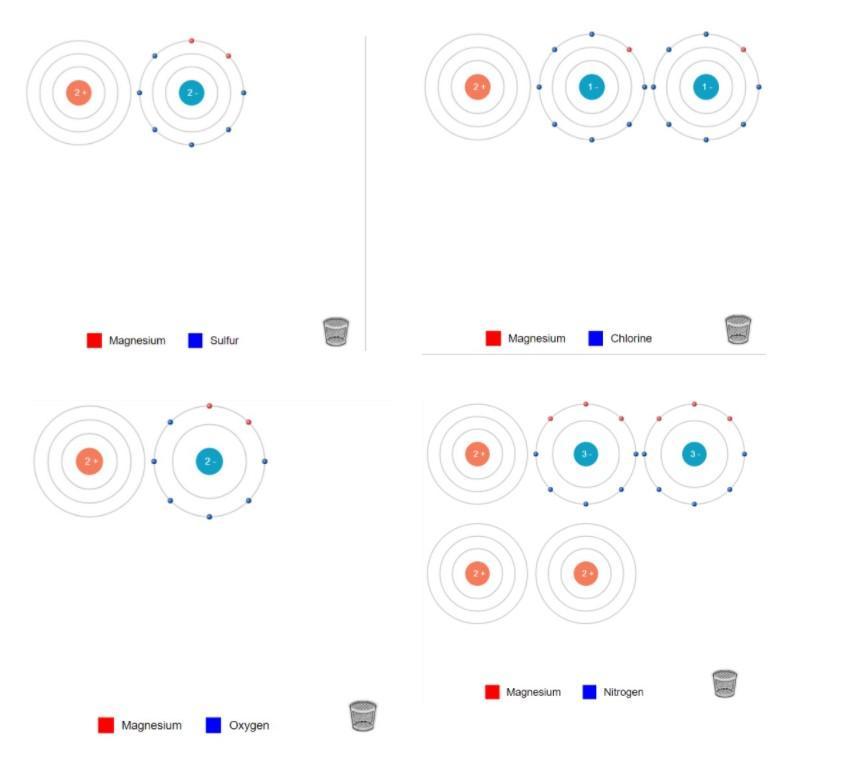
Completely describe the electrolytic cell corresponding to the following equation. (Hint: you may need to combine 2 half reactions from Table 17-1 to make one of the half reactions for this cell)
Cr2O7^2– + I^– → Cr^3+ + IO3^–
With work please COMPLETELY DESCRIBE please
Answers
The three main components of electrolytic cells are cathode, anode and electrolyte. The negatively charged electrolytic cells are cathode and the positively charged electrolytic cells are anode.
An electrolytic cell can be defined as the electrochemical device which uses the electrical energy to perform a non-spontaneous redox reaction. They are mainly used for the electrolysis of certain compounds.
Here the anode cell is:
3H₂O (l) + I⁻ (aq) → IO⁻₃ (aq) + 6e⁻ + 6H⁺ (aq)
cathode cell is:
14H⁺ (aq) + Cr₂O₇²⁻ (aq) + 6e⁻ → 2Cr³⁺ (aq) + 7H₂O (l)
To know more about electrolytic cell, visit;
https://brainly.com/question/4030224
#SPJ1
A graduated cylinder contains 50.0 ml of water. A 23.5 g piece of unknown metal is carefully dropped into the cylinder. When
the metal is completely covered with water, the water rises to the 53.4 ml mark. What is the density of the unknown piece of
metal in g/ml?
Answers
To find the density of the unknown piece of metal, we can use the formula:
Density = mass / volume.
How to find the density ?The volume of the metal can be calculated by deducting the starting water volume (50.0 ml) from the final water volume (53.4 ml) after the metal is introduced. We know the mass of the unknown metal is 23.5 g.Volume of metal = 53.4 ml - 50.0 ml = 3.4 ml.Now we can substitute the values into the density formula: Density = 23.5 g / 3.4 ml = 6.9 g/ml.Therefore, the density of the unknown piece of metal is 6.9 g/ml.To know more about density , check out :
https://brainly.com/question/1354972
#SPJ1
students investigating how gravity affects balls of different sizes, is this a good experiment?
Answers
Answer:
yea
Explanation:
Answer:1.Owen
2.By dropping the balls from the same height
3.She dropped the balls from different heights
4. Perform a second trial
Explanation: sub to technoblade plz
Experiment by hanging your first object (object 1) on the hook. Hold on to the cardboard, and position the cardboard and string so the object dangles just off the edge of the table. Then let go of the cardboard. If it moves, stop it when it reaches the edge of the table. Repeat the steps by replacing object 1 with object 2 on the hook. Finally, hang object 1 and object 2 together on the hook and repeat the steps. What do you observe when you hang different objects from the hook? What happens when two or more objects are on the hook together?
Answers
Answer:
makes now sense
Explanation:
Answer the boxes in the image

Answers
Answer: the c thingy is where you be like burgundy sauce then be like racial slair is what it mean's so the name thingy
Explanation:
What is a possible use for a genetic fingerprint?
1. To identify is someone was present at a crime scene.
II. To identify if two people are related.
III. To identify if someone is guilty of a crime.
A. I and II
B. I, II, and III
C. II and III
Answers
All of them are a possible use for a genetic fingerprint
How is drip irrigation better than canal irrigation?
Explain as well
Answers
unlike a sprinkler system that will Splash water all over the foliage of your plants a drip irrigation system will apply to water the plants where can easily be taken in by their roots
A solution with a pOH of 6.92 has an [OH−] concentration of____
Answers
Answer:
It is: 1.2x10⁻⁷
Hydroxyl ion concentration is equal to the -log[OH-]. A solution with a pOH of 6.92 has an [ OH− ] concentration of 1.2 × 10⁻⁷.
What is pOH ?The hydroxide ion ( OH- ) concentration of a solution is quantified by pOH. As a result, it can sometimes be used to determine a substance's alkalinity or even electrical conductivity.
More specifically, pOH is the hydroxide ion content's negative logarithm, which is provided by the formula:
pOH = 14 - pH.
since pH + pOH = 14, calculating pOH is simple. It is occasionally necessary to determine pOH from the concentration of hydroxide ions [OH-]. For this, to calculate concentration of OH⁻, here is the formula
pOH = -log[OH-]
Then, pOH = 14 - 6.92
= 1.2
Thus, a solution with a pOH of 6.92 has an [OH−] concentration of 1.2 × 10⁻⁷.
To learn more about pOH follow the link below;
https://brainly.com/question/17144456
#SPJ2
Please Help!! Balancing Redox Reactions Worksheet questions 4-7 (see attached)
Answers
The balanced redox reaction in the chemical reaction is given below:
40H2S + 48H+ + 16MnO4¯ ---> 5S8 + 16Mn2+ + 64H2O
Balancing the redox reaction:
Solution:
1) Half-reactions:
H2S ---> S8
MnO4¯ ---> Mn2+
2) Balance:
8H2S ---> S8 + 16H+ + 16e¯
5e¯ + 8H+ + MnO4¯ ---> Mn2+ + 4H2O
3) Make the number of electrons equal (note that there are no common factors between 5 and 16 except 1):
40H2S ---> 5S8 + 80H+ + 80e¯ <--- factor of 5
80e¯ + 128H+ + 16MnO4¯ ---> 16Mn2+ + 64H2O <---
factor of 16
4) Thus, the final answer is given below;
40H2S + 48H+ + 16MnO4¯ ---> 5S8 + 16Mn2+ + 64H2O
What is oxidation-reduction reaction?Oxidation-reduction can simply be defined as a special type of chemical reaction in which the oxidation states of the substrate change.
So therefore, the balanced redox reaction in the chemical reaction is given below:
40H2S + 48H+ + 16MnO4¯ ---> 5S8 + 16Mn2+ + 64H2O
Complete question:
Balance the following redox reaction:
MnO4¯ + H2S ---> Mn2+ + S8
Learn more about oxidation-reduction:
https://brainly.com/question/21851295
#SPJ1
Which process absorbs the greatest amount of heat?
a. the cooling of 10 g of liquid water from 100°C to 0°C.
b. the heating of 10 g of liquid water from 0°C to 100°C.
c. the freezing of 10 g of liquid water the melting of 10 g of ice.
d. the condensation of 10 g of gaseous water.
Answers
Answer:
b. the heating of 10 g of liquid water from 0°C to 100°C.
Explanation:
Hello,
In this case, we must notice a., c. and d. processes are not actually absorbing heat but releasing it since cooling, freezing and condensation are processes with negative heat sign since matter changes from a state of more energy to a state of less energy. We can prove this by realizing that freezing enthalpy of water is -6.00 kJ/mol, condensation enthalpy of eater is -40.8 kJ/mol and a change of temperature from 100 °C to 0 °C is negative.
In such a way, the only process absorbing heat is b. the heating of 10 g of liquid water from 0°C to 100°C since energy must be added to the system, or absorbed by it in order to attain the heating.
Regards.
The process having the greatest amount of heat is:
b. the heating of 10 g of liquid water from 0°C to 100°C.
Looking at all the options:The options a., c. and d. processes are not actually absorbing heat but releasing it since cooling, freezing and condensation are processes with negative heat sign since matter changes from a state of more energy to a state of less energy.
The freezing enthalpy of water is -6.00 kJ/mol, condensation enthalpy of eater is -40.8 kJ/mol and a change of temperature from 100 °C to 0 °C is negative.
So out of all the options, only process at b is a heating process thus it will absorb greatest amount of heat.
Find more information about Heat here:
brainly.com/question/13439286
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
Let's find the temperature when the pressure is equal to zero.
There are two ways of doing this, what are they? (equation line and what else?)
What is the temperature when the pressure equals zero? What is the significance of that number?
Answers
The significance of the point where the pressure drops to zero is that at that point the gases would completely show ideal behavior.
What is the gas law?The gas laws can be used to describe the behavior of the ideal gases. It is pertinent to note that the ideal gas law can strictly be applied to gases that are at a high temperature and low pressure.
We can be able to find the temperature when the pressure is equal to zero either be the use of the equation line or by experiment. In that case, we would be able to obtain the point at which the pressure drops to zero.
The significance of the point where the pressure drops to zero is that at that point the gases would completely show ideal behavior.
Learn more about idea gas:https://brainly.com/question/4194158
#SPJ1