Answers
The mass of NaCl in 44mL of a 1.6M solution is 4.1grams.
How to calculate mass?The mass of NaCl can be calculated by using the following formula;
Molarity = no. of moles ÷ volume
Molarity is the concentration of a substance in solution, expressed as the number moles of solute per litre of solution.
no of moles = 1.6M × 0.044L = 0.07moles
The mass of the sodium chloride solution can be calculated by multiplying the molar mass of the salt by the number of moles present in the solution.
The molar mass of NaCl solution can be calculated as follows;
M.M = 23g/mol + 35.5g/mol = 58.5g/mol
mass of NaCl = 58.5 × 0.07 = 4.1grams.
Therefore, 4.1grams is the mass of NaCl solution.
Learn more about molarity at: https://brainly.com/question/12127540
#SPJ1
Related Questions
Explain whether or not you expect the chaparral biome to be sensitive to the loss of a single species.
Answers
Answer:
See the answer below
Explanation:
The chaparral biome is a temperate biome with a characteristic high temperature and dryness during summer and mild rainy winters and springs. The biome can be found in relatively small amounts in the major continents of the world with its rich plant and animal diversity who have successfully adapted to the conditions of the biome.
Due to the high biodiversity of the chaparral biome, one would expect it to be resilient to the loss of a single species. The more the biodiversity of a biome or community, the more resilient such biome or community would be to the loss of species and lower the biodiversity, the more sensitive the community would be to the loss of species.
Answer:
The chaparral biome is a temperate biome with a characteristic high temperature and dryness during summer and mild rainy winters and springs. The biome can be found in relatively small amounts in the major continents of the world with its rich plant and animal diversity who have successfully adapted to the conditions of the biome.
Due to the high biodiversity of the chaparral biome, one would expect it to be resilient to the loss of a single species. The more the biodiversity of a biome or community, the more resilient such biome or community would be to the loss of species and lower the biodiversity, the more sensitive the community would be to the loss of species.
Explanation:
What most accurately describes the melting point of ionic compounds
Answers
What consist of a solid matter?
Answers
There are atoms that are close together, restricting their movement causing them to vibrate at a rapid speed.
13) Which of the following is NOT a true statement concerning what happens in all chemical
reactions
A) The ways in which atoms are joined together are changed
B) New atoms are formed as products,
C) The starting materials are named reactants.
D) The bonds of the reactants are broken and new bonds of the products are formed,
E) in a word equation representing a chemical reaction, the reactants are written on the left
and the products on the right
BARI
Answers
Please HELP!!! Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How many grams of sodium azide are required to produce 24.4 L of nitrogen gas at standard temperature and pressure? 2NaN3 --> 2Na + 3N2
47.2 g of sodium azide
106.2 g of sodium azide
1.63 g of sodium azide
0.726 g of sodium azide
Answers
Answer: 47.2 g of sodium azide
Explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number \(6.023\times 10^{23}\) of particles.
To calculate the moles, we use the equation:
\(\text{Number of moles of nitrogen}=\frac{\text{Given volume}}{\text {Molar volume}}=\frac{24.4L}{22.4L}=1.09moles\)
The balanced chemical reaction is:
\(2NaN_3\rightarrow 2Na+3N_2\)
According to stoichiometry :
3 moles of \(N_2\) are produced by = 2 moles of \(NaN_3\)
Thus 1.09 moles of \(N_2\) are produced by =\(\frac{2}{3}\times 1.09=0.73moles\) of \(NaN_3\)
Mass of \(NaN_3=moles\times {\text {Molar mass}}=0.73moles\times 65g/mol=47.2g\)
Thus 47.2 g of sodium azide are required to produce 24.4 L of nitrogen gas at standard temperature and pressure
4.What volume of hydrogen gas at STP is produced when 2.5 grams of zinc react with an
excess of hydrochloric acid?

Answers
Answer:
0.86
Explanation:
1mol of Zn has mass of 65.39g.The amount of Zn is 2.5g65.39g/mol=0.038mol.
The amount of H2 produced is the same as the amount of Zn consumed (0.038mol).
1mol of ideal gas will occupy 22.4L at STP.
The H2 will occupy 0.038mol×22.4L/mol=0.86L
.
Cassini has a mass of 2523 kg, and Saturn
has a mass of 5.68 x 1026 kg. Saturn's radius
is 54,364 km. If Cassini feels a gravitational
force of 2.980 x 104 N, how high above
Saturn's surface is it?
Rearrange F gravity Gm,m₂/r2
to solve this problem
In 10 words or fewer, how high above Saturn's surface is the Cassini
satellite?
Answers
The F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and height is 108,728 km.
What is gravity?Gravity is the amount of force that is produced by the earth to attract the object toward the surface and it doubles if the mass is double.
The height of Saturn is the duble of the radius of the given radius of 54,364 km of the planet Saturn which is 108,728 km.
Therefore, F is 2.980 x 104 N gravity Gm1 is 2523 kg m₂ is 5.68 x 1026 kg and radius 54,364 km and hight is 108,728 km.
Learn more about gravity, here:
https://brainly.com/question/4783082
#SPJ1
hi how do i do d (ii)? thanks!

Answers
A solution with nitrate(V) ion concentrations over 0.05 mg/cm3 has a molarity of around 1.61 x 10-5 mol/L.
We must first convert the amount of nitrate(V) ions in drinking water to moles per litre since the threshold concentration over which "Blue-Baby" Syndrome might manifest is 0.05 mg/cm3.
It is necessary to know the molar mass of nitrate(V) ions in order to convert from mg/cm3 to moles per litre (NO3-).
The formula below can be used to determine NO3-'s molar mass:
N: 14.01 g/mol for the atomic mass
O: 16.00 g/mol is the atomic mass (3 oxygen atoms in NO3-)
NO3-'s total molar mass is equal to 14.01 + 16.00 x 3 = 62.01 g/mol.
Let's now translate the specified concentration from mg/cm3 to moles/liter (M).
As 1 g = 1000 mg, 1 mg/cm3 is 0.001 g/cm3.
1 cm3 = 0.001 g/mL multiplied by 0.001 g/cm3
1 L/1000 mL x 0.001 g/mL = 0.001 g/L
We must change the grams into moles using the molar mass in order to get the molarity:
1.61 × 10-5 mol/L = 0.001 g/L / 62.01 g/mol
As a result, a solution with nitrate(V) ion concentrations over 0.05 mg/cm3 has a molarity of around 1.61 x 10-5 mol/L.
To know more about Blue-Baby" Syndrome:
https://brainly.com/question/31992990
#SPJ1
\( \sf{\blue{«} \: \pink{ \large{ \underline{Q\orange{U} \red{E} \green{S} \purple{TI} \pink{{ON}}}}}}\)
What is the difference between an acid and a base? Provide examples of each.
Answers
Answer:
An acid is any hydrogen-containing substance that is capable of donating a proton (hydrogen ion) to another substance. A base is a molecule or ion able to accept a hydrogen ion from an acid.
Answer:
Acids::1.Sour in taste
2. Tum blue litmus into red
3. Acids change methyl orange to red
4.Phenolphthalein remains colourless
5. Acids do not give soapy touch
6. Give hydrogen ions in solution
Bases::Bitter in taste
Bitter in tasteTurn red litmus blue Bases change methyl orange to yellowPhenolphthalein gives pink colour Soapy to touchGive hydroxyl ions in solution if it helped uh please mark me a BRAINLIEST :-))Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
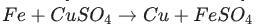
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
1. In consideration of the following acids.
HCI, H,SO, HPO, Acetic acid, Formic Acid, HF, carbonic acid, ammonium ion, water, nitric acid
a. Find conjugate bases
b. Provide complete/partial ionized equations
c. Group them as strong and weak acids
Answers
Conjugate acid-base pair is the combination of two compounds which can accept and donate hydrogen ions.
The compounds are HCI, H₂SO₄, HPO, Acetic acid, Formic Acid, HF, carbonic acid, ammonium ion, water, nitric acid.
a.
Conjugate bases of HCl is Cl⁻.
Conjugate bases of H₂SO₄ is HSO₄⁻.
Conjugate bases of HPO₄²⁻ is PO₄³⁻.
Conjugate bases of CH₃COOH is CH₃COO⁻.
Conjugate bases of formic acid is formate ion.
Conjugate bases of HF is F⁻.
Conjugate bases of carbonic acid is the bicarbonate.
Conjugate bases of ammonium ion ammonia.
Conjugate bases of water is H₃O⁺.
Conjugate bases of HNO₃ is NO₃⁻.
b.
HCl ⇄ H⁺ + Cl⁻
H₂SO₄ ⇄ 2H⁺ + SO₄²⁻
H₃PO₄ ⇄ H⁺ + PO₄³
CH₃COOH ⇄ H⁺ + CH₃COO⁻
HCOOH ⇄ H⁺ + HCOO⁻
HF ⇄ H⁺ + F⁻
H₂CO₃ + H₂O ⇄ HCO₃⁻ + H₃O⁺
Ammonia does not ionizes in water.
H₂O ⇄ H₃O⁺ + OH⁻
HNO₃ ⇄ H⁺ + NO₃⁻
c.
Strong acids: HCl, H₂SO₄, HNO₃,
Weak acids: H₃PO₄, CH₃COOH, HCOOH, HF, H₂CO₃, ammonium ion
Water is both weak and strong acid.
Learn more about conjugate bases from the link given below.
https://brainly.com/question/12883745
#SPJ1
Observe: Turn on Show molecular view, and notice the water molecules. Set the Water volume to 100 mL and the Powder mass to 20 g, and then click Play. Click Pause () after adding the powder. You should now see show some sodium acetate in the water.
What color represents the bonds between the particles of NaC2H3O2?
Click Play. Watch the animation a few times. What happens to the NaC2H3O2 bonds?
What happens to the bonds between water molecules?
Answers
Answer: how old are you
Explanation:
Silver chloride, often used in silver plating, contains 75.27% Ag.
Calculate the mass of silver chloride in grams required to make 5.2 g of silver plating.
Answers
Answer:
add
Explanation:
add 100 and 200 and that is your answer
1. What is the modern view of electrons in the quantum mechanical model?
Answers
Answer: An electron con only exist in a limited number of quantized energy levels.
Explanation:
How abundant is Polonium in the earth's crust?
Answers
Even in pitchblende, polonium is exceedingly rare: 1,000 tons of ore must be treated to yield 40 milligrams of polonium. It has a one-part-in-ten-thousandth-thousandth-thousandth-thousandth-thousandth-thousandth It is found in nature as a byproduct of the radioactive decay of uranium, thorium, and actinium.
Atomic Number: 210
How are wildfires affecting the world?
Answers
Answer:growing population,fragmentation of forests and a warming climate
Explanation:
Find the pH of the solution obtained when 0.027 L of 0.063 M benzylamine, C7H7NH2, is titrated to the equivalence point with 0.048 M hydrochloric acid. Kb for benzylamine is 4.7×10-10.
Answers
Answer:
pH = 3.12
Explanation:
C7H7NH2 + HCl ---> C7H7NH3+Cl-
moles of C7H7NH2 = 0.027 x 0.063 = 1.7 x 10^-3 mol
moles of C7H7NH2 = moles of HCl at equivalence point
1.7 x 10^-3 = 0.048 x V
V = 0.035 L
volume of HCl = 0.035 L
so C7H7NH3+Cl- salt formed = 1.7 x 10^-3 moles
salt concentration = moles / total volume
= 1.7 x 10^-3 / (0.027 + 0.035) = 0.027M
this salt formed from weak base strong acid . so pH <7
pH = 7 - 1/2 [pKb + logC]
pH = 7 -1/2 [9.33 + logC]
pH = 3.12
How many grams of Aspirin (acetylsalicylic acid) should we form in this reaction if we started with 2.08g of Salicyclic Acid?
Answers
Taking into account the reaction stoichiometry, 2.713 grams of aspirin are formed when 2.08 grams of salicyclic acid reacts.
Reaction stoichiometryIn first place, the balanced reaction is:
C₄H₆O₃ + C₇H₆O₃ (Salicyclic Acid) →C₉H₈O₄ (aspirin) + C₂H₄O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
C₄H₆O₃: 1 moleC₇H₆O₃: 1 moleC₉H₈O₄: 1 moleC₂H₄O₂: 1 moleThe molar mass of the compounds is:
C₄H₆O₃: 102 g/moleC₇H₆O₃: 138 g/moleC₉H₈O₄: 180 g/moleC₂H₄O₂: 60 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
C₄H₆O₃: 1 mole ×102 g/mole= 102 gramsC₇H₆O₃: 1 mole ×138 g/mole= 138 gramsC₉H₈O₄: 1 mole ×180 g/mole= 180 gramsC₂H₄O₂: 1 mole ×60 g/mole= 60 gramsMass of aspirin formedThe following rule of three can be applied: if by reaction stoichiometry 138 grams of salicyclic acid form 180 grams of aspirin, 2.08 grams of salicyclic acid form how much mass of aspirin?
\(mass of aspirin=\frac{2.08 grams of salicyclic acidx 180 grams of aspirin}{138 grams of salicyclic acid}\)
mass of aspirin= 2.713 grams
Then, 2.713 grams of aspirin are formed when 2.08 grams of salicyclic acid reacts.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
If 23.6 g of hydrogen gas reacts with 28.3 g of nitrogen gas, what is the maximum amount of product that can be produced?
Answers
Answer:
34.3 g NH3
Explanation:
M(H2) = 2*1 = 2 g/mol
M(N2) = 2*14 = 28 g/mol
M(NH3) = 14 + 3*1 = 17 g/mol
23.6 g H2* 1 mol/2 g = 11.8 mol H2
28.3 g N2 * 1 mol/28 g = 1.01 mol N2
3H2 + N2 ------> 2NH3
from reaction 3 mol 1 mol
given 11.8 mol 1.01 mol
We can see that H2 is given in excess, N2 is limiting reactant.
3H2 + N2 ------> 2NH3
from reaction 1 mol 2 mol
given 1.01 mol x
x = 2*1.01/1= 2.02 mol NH3
2.02 mol * 17g/1 mol ≈ 34.3 g NH3
The maximum amount of product that can be produced is 34.3 gram of NH3 will produce.
What is chemical reaction?Chemical reaction is defined as a procedure in which one or more compounds, known as reactants, are changed into one or more distinct substances, known as products.
It is defined as a process in which two or more molecules collide with the proper orientation and enough force to produce a new product.
Mass of H2 = 2x1 = 2 grams/ mole
Mass of N2 = 2 x 14 = 28 grams/ mole
Molar mass of NH3 = 14 + 1 x 3 = 17 grams/ mole
Moles of 23.6 g of H2 = 23.6 / 2 = 11.8 moles
Moles of 28.3 g of N2 = 28.3 / 28 = 1.01 moles
3H2 + N2 --> 2NH3
As per the reaction
X = 2 x 1.01 / 1 = 2.02 moles of NH3
The amount of product = 2.02 x 17 = 34.3 grams of NH3
Thus, the maximum amount of product that can be produced is 34.3 gram of NH3 will produce.
To learn more about chemical reaction, refer to the link below:
https://brainly.com/question/3461108
#SPJ2
Question 1
4.52 moles of Propane in a canister has a volume of 21.0 L and a temperature of 298.0 K what is the pressure in kPa? kPa
R=8.31 L * KPa/mole * K
Round your final answer to the nearest 10th (one decimal point)
Question 2
What is the temperature, in K, of 1.60 mol of hydrogen gas that has a volume of 42.58 liters under 950.2 mmHg of pressure? K
R=8.31 L * KPa/mole * K
101.3 KPa = 760.0 mmHg
Round your final answer to the nearest 10th (one digit after the decimal point).
Answers
Answer:
(1). The pressure is 533 KPa.
(2). The temperature is 406 K.
Explanation:
Given that,
Number of moles = 4.52 moles
Volume = 21.0 L
Temperature = 298 K
(1). We need to calculate the pressure
Using ideal equation of gas
\(PV=nRT\)
\(P=\dfrac{nRT}{V}\)
Where, T = temperature
R = gas constant
V = volume
n = number of moles
Put the value into the formula
\(P=\dfrac{4.52\times8.31\times298}{21.0}\)
\(P=533.0\ KPa\)
(2). Number of moles = 1.60 moles
Volume = 21.0 L
Temperature = 298 K
We need to calculate the temperature
Using ideal equation of gas
\(PV=nRT\)
\(T=\dfrac{PV}{nR}\)
Put the value into the formula
\(T=\dfrac{126.68293\times42.58}{1.60\times8.31}\)
\(T=406\ K\)
Hence, (1). The pressure is 533 KPa.
(2). The temperature is 406 K.
4. How many grams is 3 moles of H₂O?
Answers
Answer:
1.67
Explanation:
Mass÷mr=moles
3÷18=1.67
c) Discuss precision and Accuracy as they relate to types of errors.
what is the answer
Answers
Precision relates to the consistency and reproducibility of measurements, while accuracy reflects how close measurements are to the true value.
Precision and accuracy are two important concepts in the context of errors in measurements. While they both pertain to the quality of data, they refer to different aspects.
Precision refers to the degree of consistency or reproducibility in a series of measurements. It reflects the scatter or spread of data points around the average value. If the measurements have low scatter and are tightly clustered, they are considered precise. On the other hand, if the measurements have a high scatter and are widely dispersed, they are considered imprecise.
Accuracy, on the other hand, refers to the closeness of measurements to the true or target value. It represents how well the measured values align with the actual value. Accuracy is achieved when measurements have a small systematic or constant error, which is the difference between the average measured value and the true value.
Errors in measurements can be classified into two types: random errors and systematic errors.
Random errors are associated with the inherent limitations of measurement instruments or fluctuations in the measurement process. They lead to imprecise data and affect the precision of measurements. Random errors can be reduced by repeating measurements and calculating the average to minimize the effect of individual errors.
Systematic errors, on the other hand, are caused by consistent biases or inaccuracies in the measurement process. They affect the accuracy of measurements and lead to a deviation from the true value. Systematic errors can arise from factors such as instrumental calibration issues, environmental conditions, or experimental techniques. These errors need to be identified and minimized to improve the accuracy of measurements.
In summary, precision refers to the degree of consistency or reproducibility of measurements, while accuracy refers to the closeness of measurements to the true value. Random errors affect precision, while systematic errors affect accuracy. To ensure high-quality measurements, both precision and accuracy need to be considered and appropriate techniques should be employed to minimize errors.
Know more about Precision here:
https://brainly.com/question/30461151
#SPJ8
Fifteen kg of iron (lll) oxide was used in a reaction to produce iron.calculate the mass of iron produced in this reaction
Answers
10.50 kg of iron will be produced from 15 kg of iron (III) oxide.
What is mass?The amount of matter in an item is measured by its mass, which is a fundamental physical quantity. It is a scalar amount that is measured in kilograms (kg) or grams (g). Regardless of an object's location or the force pressing against it, its mass always remains constant.
How do you determine it?Iron (III) oxide and elemental iron react chemically in the following balanced chemical equation:
2 Fe2O3+ 3 C = 4 Fe + 3 CO2
Due to the reaction between 2 moles of Fe2O3 and 4 moles of Fe, the mole ratio of Fe2O3 to Fe is either 2:4 or 1:2.
The amount of iron created from 15 kg of Fe2O3 can be calculated using this mole ratio:
Fe2O3 = 2 moles of Fe per mole.
Molecular weight of Fe2O3 is 159.69 g/mol.
Fe2O3 has a mass of 15 kg and a density of 15,000 g/mol, or 94.00 moles.
We can figure out how many moles of Fe were produced using the mole ratio of 1:2:
We can figure out how many moles of Fe were produced using the mole ratio of 1:2:
2 moles of Fe for each mole of Fe2O3 94.00 moles of Fe2O3 multiplied by (2 moles of Fe for each mole of Fe2O3) results in 188.00 moles of Fe.
The molar mass of Fe can then be used to convert the moles of iron to mass as follows:
Fe's molecular weight is 55.85 g/mol.
188.00 moles of Fe produced at a rate of 55.85 g/mol result in a mass of 10,499.80 g or 10.50 kg.
Hence, 10.50 kg of iron will be produced from 15 kg of iron (III) oxide.
To know more about mass, visit:
brainly.com/question/19694949
#SPJ1
HBr can be added to an alkene in the presence of peroxides, R-O-O-R. What role do peroxides play in this reaction
Answers
Answer:
The peroxide initiates the free radical reaction
Explanation:
The addition of HBr to alkene in the presence of peroxides occurs via a free radical mechanism.
The organic peroxide acts as the initiator of the free radical reaction. The organic free radical interacts with HBr to produce a bromine free radical which now interacts with the alkene and the propagation steps continue until it is terminated by the coupling of two free radicals.
The peroxide effect leads to anti-Markovnikov addition.
If I add 50 mls of water to 300 mls of 0.6M KNO3 solution, what will be the molarity of the diluted solution?
Answers
Answer:
\(M_2=0.51M\)
Explanation:
Hello,
In this case, for this dilution process, we understand that the moles of the solute (potassium nitrate) remain unchanged upon the addition of diluting water. However, the resulting or final volume includes the added water as shown below:
\(V_2=300mL+50mL=350mL\)
In such a way, we are able to relate the solution before and after the dilution by:
\(V_1M_1=V_2M_2\)
Hence, we solve for the final molarity as:
\(M_2=\frac{M_1V_1}{V_2}=\frac{0.6M*300mL}{350mL}\)
Best regards.
\(M_2=0.51M\)
even one or two crystals of copper sulphate can make its solution in water coloured blue. why
Answers
this also may happen because of the water molecules that get attached
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
A beam of x-ray of wavelength 0.071 nm is diffracted by (110) plane of rock salt with lattice constant (a) 0.28nm. find the glancing angle for the second order diffraction
Answers
The glancing angle for the second order diffraction of X-rays with a wavelength of 0.071 nm diffracted by the (110) plane of rock salt with a lattice constant of 0.28 nm can be calculated using Bragg's law:
2d sinθ = nλ
where d is the interplanar spacing of the (110) plane, n is the order of the diffraction, λ is the wavelength of the X-rays, and θ is the glancing angle.
For the second order diffraction (n = 2), we have:
2d sinθ = 2λ
The interplanar spacing of the (110) plane can be calculated using the formula:
d = a/√(h^2 + k^2 + l^2)
where a is the lattice constant, and h, k, and l are the Miller indices of the (110) plane. For rock salt, h = 1, k = 1, and l = 0 for the (110) plane.
Substituting the values given in the problem, we get:
d = 0.28 nm / √(1^2 + 1^2 + 0^2) = 0.14 nm
Substituting the values for d, n, and λ, we get:
2(0.14 nm) sinθ = 2(0.071 nm)
Simplifying, we get:
sinθ = 0.51
Taking the inverse sine, we get:
θ = 30.2 degrees
Therefore, the glancing angle for the second order diffraction is approximately 30.2 degrees.
altose (C12H22O11) solutions are commonly used in chemistry and biomedical labs. The molar masses of the elements in maltose are listed below. C: 12.01 g/mol, H: 1.008 g/mol, O: 16.00 g/mol. Describe how to prepare 1.000 L of a 0.500 M maltose solution.
Answers
To prepare a 1.000 L of 0.500 M maltose solution, we first need to calculate the mass of maltose required and then dissolve it in water to make the final solution.
Calculate the mass of maltose needed. The formula for molarity is M = moles/L. Rearrange the formula to solve for moles: moles = M * L. In this case, moles = 0.500 M * 1.000 L = 0.500 moles.
Next, calculate the molar mass of maltose. The formula for molar mass is M = mass/moles. Rearrange the formula to solve for mass: mass = M * moles.
The molar mass of maltose is (12.01 g/mol * 12) + (1.008 g/mol * 22) + (16.00 g/mol * 11) = 342.30 g/mol. Therefore, the mass of maltose needed is 342.30 g/mol * 0.500 moles = 171.15 g.
Measure out 171.15 g of maltose using a balance. Then dissolve the maltose in a small amount of distilled water in a volumetric flask. Add more distilled water until the volume reaches 1.000 L.
Mix the solution thoroughly to ensure that the maltose is fully dissolved.
Your 1.000 L of 0.500 M maltose solution is now ready for use in your experiment.
To know more about maltose solution refer here:
https://brainly.com/question/30568906#
#SPJ11
Is silver bromide a compound or elementary substance?
Answers
Answer:Silver bromide (AgBr), an important component of photographic film, is, like silver chloride and iodide, light sensitive. Traces of potassium bromate (KBrO3) ...
Explanation:
Answer:
Formula and structure: The chemical formula of silver bromide is AgBr and its molar mass is 187.77 g/mol. It is an inorganic compound made up of the silver metal (Ag) and the bromine atom (Br), held together through a polar covalent bond which has a strong ionic character.
I hope this helps friend! :D