Answers
Answer:
1.62 g of Al contain the same number of atoms as 6.35 g of cadmium have.
Explanation:
Given data:
mass of cadmium = 6.35 g
Number of atoms of aluminum as 6.35 g cadmium contain = ?
Solution:
Number of moles of cadmium = 6.35 g/ 112.4 g/mol
Number of moles of cadmium = 0.06 mol
Number of atoms of cadmium:
1 mole = 6.022×10²³ atoms of cadmium
0.06 mol × 6.022×10²³ atoms of cadmium/ 1mol
0.36×10²³ atoms of cadmium
Number of atoms of Al:
Number of atoms of Al = 0.36×10²³ atoms
1 mole = 6.022×10²³ atoms
0.36×10²³ atoms × 1 mol /6.022×10²³ atoms
0.06 moles
Mass of aluminum:
Number of moles = mass/molar mass
0.06 mol = m/ 27 g/mol
m = 0.06 mol ×27 g/mol
m = 1.62 g
Thus, 1.62 g of Al contain the same number of atoms as 6.35 g of cadmium have.
Related Questions
If 5.0 mL of 0.10 M NaOH is added to 50. mL of 0.10 M HCI, what will be the resulting
pH of the solution?
Round your answer to two decimal places.
Provide your answer below:
PH
Answers
Note that NaOH and HCl are the reactants of the reaction. Since they’re a strong acid and base, they would completely dissociate, meaning the molarity of H+ is equal to the molarity of HCl (same applies for NaOH and OH-) which also means the moles are the same.
Solve for the moles after the reaction has fully occurred then use the total volume (since this is the end of the reaction, volumes are fully mixed) and find the new molarity of H+ since our NaOH was our limiting reactant.
The plug the molarity of H+ into the formula pH = -log[H+] and get the pH of 1.09

Answer:
1.09
Explanation:
Keep in mind that the volume of the solution changes during this titration, so to compute the amount of hydronium that is neutralized during this addition of base (in order to calculate the final pH of the solution), we must calculate the moles of all species in solution initially present. Because both NaOH and HCl ionize completely:initial mol OH−=mol NaOH=(0.0050 L)(0.10 molL)=0.00050 mol OH−initial mol H3O+=mol HCl=(0.050 L)(0.10 molL)=0.0050 mol H3O+The acid is in excess, so all of the OH− present will neutralize an equivalent amount of H3O+, forming water. Thus we simply subtract the moles of hydroxide from the moles of hydronium in solution to find the resultant moles of H3O+ after this neutralization:final mol H3O+= initial mol H3O+−initial mol OH−final mol H3O+=0.0050 mol−0.00050 mol=0.0045 mol H3O+We now calculate the total volume of the solution by adding the volumes of acid and base initially combined: 0.050 L+0.0050 L=0.055 LTo get [H3O+], we divide the final moles of hydronium by the final solution volume:[H3O+]=final mol H3O+ total volume=0.0045 mol0.055 L≈0.08181molLFinally, to find pH:pH=−log[H3O+]=−log(0.08181)=1.09Since the hydronium concentration is only precise to two significant figures, the logarithm should be rounded to two decimal places.
A student wishes to prepare 25mL of 0.1M NaOH from 6M NaOH.
How many mL of 6M NaOH need to be placed in a 25.00mL volumetric flask for dilution to generate
25mL of a 0.1M solution?
Answers
Answer:
Na(OH)4
Explanation:
Look at the charges and add them up
Na(OH) Na(OH)
please help me ASAAAAAAAAAAAP
Identify the Arrhenius acid and the Arrhenius base in this reaction.
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Question 35 options:
Na2SO4(acid), 2H2O(base)
H2SO4(acid), NaOH(base)
NaOH(acid), Na2SO4(base)
H2SO4(acid), Na2SO4(base)
Answers
Answer:
H2SO4(acid), NaOH(base)
Explanation:
What forces typically hold ions together ?
Answers
Ions are atoms or molecules that have an unequal number of protons and electrons, resulting in a net electrical charge. When ions come into close proximity to each other, they can be attracted to or repelled by each other due to electrostatic forces.
There are two main types of electrostatic forces that can hold ions together:
1. Ionic bonds: These are electrostatic forces of attraction between positively charged metal ions and negatively charged non-metal ions. This type of bond typically forms between metal and non-metal ions that have a large difference in electronegativity, resulting in a transfer of electrons from the metal atom to the non-metal atom. The resulting oppositely charged ions are then held together by strong electrostatic forces, forming a stable ionic compound.
2. Electrostatic attraction between oppositely charged ions: This type of electrostatic attraction can occur between any two ions that have opposite charges, regardless of whether they are metals or non-metals. For example, when a positive ion (cation) and a negative ion (anion) are brought close together, they can be attracted to each other by electrostatic forces. The strength of the attraction depends on the charges of the ions and the distance between them.
Overall, the forces that hold ions together are typically electrostatic in nature and are a result of the attraction between opposite charges.
PLEASE HELP!!!
How many calories are in 4,180 joules?
Answers
Answer:
To convert joules to calories, you can use the conversion factor:
1 calorie = 4.184 joules
To find out how many calories are in 4,180 joules, divide the given value by the conversion factor:
4,180 joules / 4.184 joules per calorie = 0.9 calories (approximately)
Therefore, there are approximately 0.9 calories in 4,180 joules.
Axel decided to start working out three times a week to stay healthy. Which type of disease prevention is being described?
Answers
Answer:
A
Explanation:
behavior modification
What mass of NaCl is in 1.25L of 0.1035M solution?
Answers
Answer:
Explanation:
To determine the mass of NaCl in 1.25L of 0.1035M solution, we can use the formula:
mass = concentration x volume x molar mass
where concentration is in molarity (M), volume is in liters (L), and molar mass is in grams per mole (g/mol).
The molar mass of NaCl is 58.44 g/mol.
Plugging in the given values, we get:
mass = (0.1035 M) x (1.25 L) x (58.44 g/mol)
mass = 7.3188 g
So, there is approximately 7.3188 grams of NaCl in 1.25L of 0.1035M solution.
Identify the oxidizing and reducing agents in the following: H2S(aq) + Cl2(g) -> S(s) + 2HCI (aq)
Answers
The oxidizing and reducing agent in the above redox reaction are hydrogen sulphide (H2S) and Chlorine (Cl) respectively.
What is an oxidizing and reducing agent?An oxidizing agent is any substance that oxidizes, or receives electrons from another substance and as a result, becoming reduced.
On the other hand, a reducing agent is any substance that reduces or donates electrons to another and as a result becomes oxidized.
According to this reaction; H2S(aq) + Cl2(g) -> S(s) + 2HCI (aq)
H2S accepts electrons from Cl2 and becomes reduced to SCl2 donates electrons to H2S and becomes oxidized to HClTherefore, the oxidizing and reducing agent in the above redox reaction are hydrogen sulphide (H2S) and Chlorine (Cl) respectively.
Learn more about oxidizing agent at: https://brainly.com/question/10547418
#SPJ1
41
The flow of rivers is affected by natural environmental conditions and human activities.
Which of the following human activities would result in the most impact on the flow of
water in a river?
A. The release of water from a water treatment plant into the river
B. The development of a shopping center along the riverbank
C. Harvesting trees from a forest along the riverbank
D. Constructing a dam in the river
Answers
B. The development of a shopping center along the riverbank is the following human activities would result in the most impact on the flow of water in a river
How do factors can impact way water flows?By building dams to store water and by taking water for household, commercial, and agricultural uses, humans actively alter the dynamics of the water cycle. Water demand and supply are expected to be even more impacted by climate change.
Human development, including population increase, reliance on fossil fuels, urbanisation, intercontinental trade, and industrial and agricultural emissions, is typically the cause of environmental changes in rivers.
Acid rain allows nitrous oxide and sulphur dioxide from industries and power plants to infiltrate river systems. In certain places, sewage and wastewater are dumped into rivers. All creatures, from algae to vertebrates, are impacted by pollution when the pH of the water is lowered. As pH falls, biodiversity declines.
learn more about human activities refer
https://brainly.com/question/25213840
#SPJ9
a bullet travels at 500 m/s. How long will it take a bullet to go 1000m?
Answers
Answer:
2 seconds
Explanation:
So we set up the equation
500s = 1000, divide 1000 by 500
1000/500 = 2
So s = 2, it will take two seconds for the bullet to travel 1000m
how many moles of oxygen are needed to react completely with 17.2 moles of aluminum
Answers
Answer:As you have asked, I will not do your homework for you but will outline the reasoning…
Find out the atomic weight of all the species involved in the problem. Three decimals is more than sufficient.
Look at the periodic table of the elements and other resources (notes!) as necessary to determine the expected valence of the species
Once you know how many of these go with those, you are cruising…
You already know that 4Al+3O2→2Al2O3
So, you are in a great position to determine the number of moles of O2 , as you know you have 14.8 mol of Al and the ratio of combination is always 4 molecules.
Explanation:
please help, I'm giving brainliest. Explain the term'wear acid'.
Answers
Weak acids are acids that don't completely dissociate in solution. In other words, a weak acid is any acid that is not a strong acid. The strength of a weak acid depends on how much it dissociates: the more it dissociates, the stronger the acid.
What is a sedimentary rock?
Answers
Answer:
a rock that is composed of compacted sediment, sand, pieces of other rock, or minerals.
Explanation:
Answer:
Sedimentary rocks are types of rock that are formed by the accumulation or deposition of mineral or organic particles at Earth's surface, followed by cementation. Sedimentation is the collective name for processes that cause these particles to settle in place.
Explanation:
I hope this helps
Rank the the following parts of the EMS from shortest wavelength to longest wavelength. Input your answer as a 7 digit number (ex. 7654321)
1. visible light
2. ultraviolet
3. gamma rays
4. radio waves
5. x rays
6. microwaves
7. infrared
Answers
The order of the components of the electromagnetic spectrum from shortest wavelength to longest is as follows: 3-5-2-1-7-6-4.
What is electromagnetic spectrum?Electromagnetic spectrum is the entire range of wavelengths of all known electromagnetic radiations extending from gamma rays, to X-rays through visible light, infrared, to radio waves.
The components of the electromagnetic spectrum from order of shortest to longest wavelength is as follows;
Gamma raysX-raysUV radiationVisible lightInfrared lightMicrowavesRadio wavesThis suggests that gamma rays have the shortest wavelength while radio waves have the longest wavelength.
Learn more money electromagnetic spectrum at: https://brainly.com/question/23727978
#SPJ1
4- The standard potential of cell: Sn/Sn²+||Cr³+/Cr is −0.60V.what is the standard
reduction potential of the Cr³+/Crelectrode? Es = -0.14V
Sn²+
(b) +0.74V
(c) -0.88V
(d) -0.74V
(a) +0.88V
Answers
The standard reduction potential of the Cr³+/Cr electrode is -0.46V. None of the option is correct.
To determine the standard reduction potential of the Cr³+/Cr electrode, we can use the Nernst equation, which relates the standard reduction potential to the cell potential under non-standard conditions. The Nernst equation is given by:
E = E° - (0.0592/n) * log(Q)
where E is the cell potential, E° is the standard reduction potential, n is the number of electrons transferred in the half-reaction, and Q is the reaction quotient.
In this case, we have the standard potential of the cell as −0.60V. We know that the standard reduction potential of the Sn/Sn²+ electrode is -0.14V. Therefore, the reduction potential of the Cr³+/Cr electrode can be calculated as:
E = -0.60V - (-0.14V)
E = -0.60V + 0.14V
E = -0.46V
Therefore, the standard reduction potential of the Cr³+/Cr electrode is -0.46V.
For more such question on standard reduction potential visit;
https://brainly.com/question/31482299
#SPJ8
The acid ionization constant, Ka, for propanoic acid, C2H5COOH, is 1.3x10-5.(a) Calculate the hydrogen ion concentration, [H+], in a 0.20-molar solution of propanoic acid.(b) Calculate the percentage of propanoic acid molecules that are ionized in the solution in (a).(c) What is the ratio of the concentration of propanoate ion, C2H5COO-, to that of propanoic acid in a buffer solution with a pH of 5.20?(d) In a 100.-milliliter sample of a different buffer solution, the propanoic acid concentration is0.35-molar and the sodium propanoate concentration is 0.50-molar. To this buffer solution,0.0040 mole of solid NaOH is added. Calculate the pH of the resulting solution
Answers
(a) The hydrogen ion concentration in the solution is [H+] = 1.14x10^-3 M. (b) 0.57%. (c) The ratio of the concentration of propanoate ion to that of propanoic acid in the buffer solution is 2.68.
(a) The balanced equation for the ionization of propanoic acid is:
C2H5COOH + H2O ⇌ C2H5COO- + H3O+
The equilibrium expression for this reaction is:
Ka = [C2H5COO-][H3O+] / [C2H5COOH]
At equilibrium, the concentration of propanoic acid that has ionized to form propanoate ion and hydronium ion is equal to the concentration of propanoic acid that has not ionized, so we can assume that [C2H5COO-] ≈ [H3O+]. Let x be the concentration of hydronium ion in the solution. Then the equilibrium expression becomes:
Ka = x^2 / (0.20 - x)
Solving for x, we get:
x = sqrt(Ka * (0.20 - x)) = sqrt(1.3x10^-5 * 0.20) = 1.14x10^-3 M
Therefore, the hydrogen ion concentration in the solution is [H+] = 1.14x10^-3 M.
(b) The percentage of propanoic acid molecules that are ionized in the solution is given by:
% ionization = [H3O+] / [C2H5COOH] x 100%
% ionization = (1.14x10^-3 / 0.20) x 100% = 0.57%
(c) The pH of a buffer solution can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([C2H5COO-] / [C2H5COOH])
At pH 5.20, the hydronium ion concentration is 10^-5.20
= 6.31x10^-6 M.
Using the equilibrium expression for propanoic acid and the fact that [C2H5COO-] + [C2H5COOH] = total buffer concentration,
we can solve for the ratio of the concentrations of propanoate ion to propanoic acid:
Ka = [C2H5COO-][H3O+] / [C2H5COOH]
[C2H5COO-] = Ka[C2H5COOH] / [H3O+]
[C2H5COO-] = (1.3x10^-5)([C2H5COOH]) / (6.31x10^-6)
[C2H5COO-] / [C2H5COOH]
= 2.68
Therefore, the ratio of the concentration of propanoate ion to that of propanoic acid in the buffer solution is 2.68.
(d) When solid NaOH is added to the buffer solution, it reacts with the propanoic acid to form propanoate ion and water:
C2H5COOH + NaOH → C2H5COO- + H2O + Na+
The number of moles of propanoic acid that react with NaOH is equal to the number of moles of NaOH that were added. The new concentration of propanoic acid is:
0.35 M - (0.0040 mol / 0.100 L) = 0.346 M
The new concentration of propanoate ion is:
0.50 M + (0.0040 mol / 0.100 L) = 0.54 M
The new concentration of hydronium ion can be calculated using the equilibrium expression.
Learn more about equilibrium here:
https://brainly.com/question/3920294
#SPJ4
2. A company makes mixtures of acetic acid and water such that the acetic acid is 15% of the total mass (weight) of the mixture. Let A be an unspecified number of grams of acetic acid, which can vary and let W be the corresponding number of grams of water in this type of mixture.
An equation that relates A and W is A = (3/17) W.
Answers
The equation that relates A and W, considering the desired 15% acetic acid concentration, is 3W = 2.55M.
The equation A = (3/17)W represents the relationship between the mass of acetic acid (A) and the mass of water (W) in the mixture. It states that the mass of acetic acid is equal to three seventeenths (3/17) of the mass of water.
Since the company wants the acetic acid to be 15% of the total mass of the mixture, we can set up another equation to represent this requirement. Let M be the total mass of the mixture. The mass of acetic acid (A) is 15% of the total mass, so we have A = 0.15M.
Now we can substitute A in terms of W from the first equation into the second equation: (3/17)W = 0.15M. We can simplify this equation by multiplying both sides by 17 to get 3W = 2.55M.
This equation allows the company to calculate the mass of water (W) required for a given mass of acetic acid (A) to maintain the desired concentration in the mixture.
For such more questions on concentration
https://brainly.com/question/26175405
#SPJ8
write five characteristics of good government?
Answers
Explanation:
giving equal opportunity,making good governance, maintaining peace and harmony among people, other try to write by your self
Which of the following statements about density is NOT true?
Group of answer choices
Density can be used to determine the identity of a substance.
Density is an extensive property of matter.
Density is an intensive property of matter.
Density is the ratio of two extensive properties.
Answers
The statement that is not true about density is that density is an extensive property.
WHAT IS AN EXTENSIVE PROPERTY: Extensive property is the property of a substance that is dependent on the amount of the substance. Examples of extensive properties are mass, volume etc. Density is an intensive property because it is not dependent on the amount of the substance rather than the type of substance. Intensive properties can be used to determine the identify of a substance. Density is a ratio of two extensive properties i.e. mass and volume.Therefore, the statement that is not true about density is that density is an extensive property.
Learn more about density at: https://brainly.com/question/952755
25 points and I’ll mark as brainliest!!! Tasks are in the picture.

Answers
5. There are 5.67 grams of HBr dissolved in 700 mL of the solution with pH of 2.
6. There are 1000 times more hydrogen ions in a solution with a pH of 3 than in a solution with a pH of 6.; option D.
How many grams of HBr are dissolved in 700 mL in the solution with pH of 2?The grams of HBr dissolved in 700 mL of a solution with pH of 2 is determined as follows;
pH = -log[H+]
2 = -log[H+]
[H+] = 10⁻² mol/L
HBr is a strong acid and dissociates completely in water to give H+ and Br- ions as follows:
HBr → H+ + Br-
The concentration of HBr in the solution will then be equal to the concentration of H+ ions.
[HBr] = 10⁻² mol/L
The mass of HBr is then determined using the formula:
mass = concentration x volume x molar massThe molar mass of HBr is 80.91 g/mol.
mass = 10⁻² mol/L x 0.7 L x 80.91 g/mol
mass = 5.67 g
Learn more about pH at: https://brainly.com/question/172153
#SPJ1
Examine the Lewis dot structure of propene, C3H6 , and answer the following questions. A central carbon atom is bonded to one hydrogen atom through a single bond, one carbon atom thorugh a single bond, and one carbon atom through a double bond. This carbon atom is labeled B. The double bonded outer carbon is bonded to two hydrogen atoms through single bonds and is labeled C. The single bonded outer carbon is bonded to three hydrogen atoms through single bonds and is labeled A. How many groups of electrons are around carbon atom B in propene
Answers
Answer:
The central carbon atom, B, in propene has 3 electron group around it.
Explanation:
Considering the Lewis dot structure of propene, the following observations were made:
1. The central carbon atom has four valence electrons
2. Two of the valence shell electrons are shared with carbon atom C, which contributes two electrons as well to form a covalent double bond.
3. Another valence electron of the central carbon atom is shared with a hydrogen atom which contributes one electron as well to form a covalent single bond.
4. The remaining valence electron is shared with another carbon atom, A, in a covalent single bond with the carbon atom A, also contributing an electron for the single bond.
5. The double bond to carbon C, the single bond to a hydrogen atom and the single bond to carbon atom, A, constitutes Three electron groups around the central carbon atom, B.
Therefore, the central carbon atom, B, in propene has 3 electron group around it.
Looking at the Lewis dot structure of propene as shown, there are three electron groups that surround the central carbon atom.
According to the valence shell electron pair repulsion theory (VSEPR) the shape of a molecule depends on the number of electron pairs that surround the central atom in the molecule. These electron pairs could be lone pairs or bond pairs.
In propene, we have three electron groups that surround the central carbon atom labelled B. This is because, double and triple bonds are counted as a single electron group.
Learn more: https://brainly.com/question/14191541

Classify each of the ions as monoatomic or polyatomic.
Help me out

Answers
\(Na^+\), \(Ca^2^+\), \(F^-\), and \(S^2^-\) are monoatomic ions, while \(Cr^2^+\), \(Hg^2^+\), \(ClO^-\), \(OH^-\), NO3-, and ClO3- are polyatomic ions. H₂O is not an ion; it is a neutral molecule.
Monoatomic ions are ions formed from a single atom, while polyatomic ions are ions formed from a group of atoms bonded together. Let's classify each of the ions you provided:
Monoatomic ions:
- Na+ (sodium ion)
- Ca2+ (calcium ion)
- F- (fluoride ion)
- S2- (sulfide ion)
Polyatomic ions:
- Cr2+ (chromium(II) ion) - This ion should be Cr2+, indicating a two-positive charge on the chromium ion. It is typically found in compounds with ligands to stabilize its charge.
- Hg2+ (mercury(II) ion) - This ion is a polyatomic ion due to the presence of two mercury atoms bonded together.
- H₂O (water molecule) - This is not an ion; it is a neutral molecule consisting of two hydrogen atoms bonded to one oxygen atom.
- ClO- (hypochlorite ion) - This is a polyatomic ion consisting of chlorine and oxygen atoms.
- OH- (hydroxide ion) - This is a polyatomic ion consisting of one oxygen and one hydrogen atom.
- NO3- (nitrate ion) - This is a polyatomic ion consisting of one nitrogen and three oxygen atoms.
- ClO3- (chlorate ion) - This is a polyatomic ion consisting of one chlorine and three oxygen atoms.
for more questions on polyatomic
https://brainly.com/question/15599646
#SPJ8
Monoatomic ions consist of a single atom, while polyatomic ions consist of more than one atom. For example, Na+ is monoatomic, while NO3- is polyatomic.
Explanation:To classify ions as either monoatomic or polyatomic, you need to know the nature of these ions. Monoatomic ions are ions made up of a single atom, while polyatomic ions are made of more than one atom. For example, Na+ is a monoatomic ion because it consists of a single sodium atom. On the other hand, NO3- is a polyatomic ion because it consists of one nitrogen atom and three oxygen atoms.
Learn more about Classifying Ions here:https://brainly.com/question/33929059
#SPJ11
Which 3 laws combine to make the Combined Gas Law?
Answers
Answer:
Boyle's Law, Charles' Law, and Gay-Lussac's Law
Explanation:
Answer:
Boyle's Law, Charles's Law, and Gay-Lussac's Law
Explanation:
What is the mass of a rectangular piece of copper 24.4cm x 11.4 cm x 7.9 cm? The density of copper is 8.92g/cm3.
Answers
The mass of the rectangular piece of copper is 18,869 g (approx).In conclusion, the mass of a rectangular piece of copper with dimensions 24.4cm x 11.4 cm x 7.9 cm and a density of 8.92 g/cm³ is 18,869 g (approx.).
The given dimensions of the rectangular piece of copper are:Length = 24.4 cmWidth = 11.4 cmHeight = 7.9 cmThe formula to calculate the mass of an object is given by;
Mass = Density x Volume
Here, the density of copper is given as 8.92 g/cm³.
Therefore, the first step is to calculate the volume of the rectangular piece of copper.The formula to calculate the volume of a rectangular object is given by:
Volume = Length x Width x Height
So,Volume = 24.4 cm x 11.4 cm x 7.9 cm= 2115.432 cm³Now we will use the mass formula:
Mass = Density x Volume= 8.92 g/cm³ x 2115.432 cm³= 18,869.27824 g= 18,869 g (approx.)
For more such questions on copper
https://brainly.com/question/29176517
#SPJ8
12: from the following list, decide whether the following are Arrhenius bases or acids.•HCl•NaOh•HBr•H2SO4•NH4OH•H3PO4•HNO3•LiOH•Ba(OH)2•CsOH•HF•KOH
Answers
Answer:
The Arrhenius acids in the list are:
HCl, HBr , H2SO4 , H3PO4 , HNO3 , HF
The Arrhenius bases are:
NaOH , NH4OH , LiOH , Ba(OH)2 , CsOH and KOH
Explanation:
An Arrhenius acid increases the concentration of H+ ions while an Arrhenius acid increases the concentration of OH- ions
Thus, an Arrhenius acid would yield H+ ions on ionization while an Arrhenius base will yield OH- ions on ionization
The Arrhenius acids in the list are:
HCl, HBr , H2SO4 , H3PO4 , HNO3 , HF
The Arrhenius bases are:
NaOH , NH4OH , LiOH , Ba(OH)2 , CsOH and KOH
balance the chemical reaction ___ Al + ___ FeO → ___ Al2O3 + ___ Fe
Answers
Answer:
2Al+3FeO→Al2O3+3Fe
Explanation:
Basically, to get this answer, you need to balance the amount of Aluminum to the ammount on the other side which is 2 so you need 2 Al to balance the reaction correct, next you move on to the amount of Oxygen in the reaction, there are three Oxygen’s on the right and one on the left, so you need 3 FeO in order for the Oxygens to be balanced. Now that the Iron is unbalanced on both sides, you need 3 Fe (on the right) in order for the equation to be balanced.
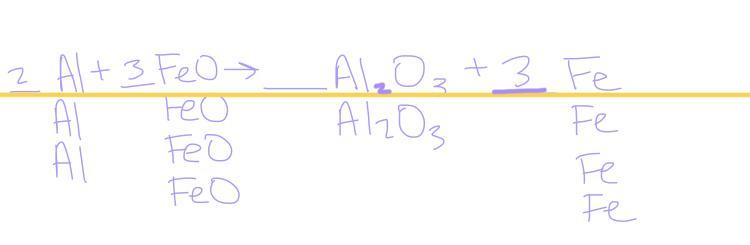
The balanced chemical reaction is \(2Al+3FeO\) → \(Al_2O_3+3Fe\)
What is a balanced chemical reaction?A balanced chemical equation occurs when the number of atoms involved on the reactants side is equal to the number of atoms on the products side.
To balance a reaction means to make the number of atoms the same on both the reactants and products sides. To do so, coefficients need to be added to the chemical equation.
Hence, the balanced the chemical reaction is \(2Al+3FeO\) → \(Al_2O_3+3Fe\).
Learn more about the balanced chemical reaction here:
https://brainly.com/question/15196081
#SPJ2
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
draw the lewis dot structure of C4H8N2O3
Answers
Explanation:
Refer to the attachment
Extrashots:-
C4H8N2O3 is called asparagine .

a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
What is the limiting reagent when a 2.00 g sample of ammonia is mixed with 4.00 g of oxygen?
Answers
Answer:
Ammonia is limiting reactant
Amount of oxygen left = 0.035 mol
Explanation:
Masa of ammonia = 2.00 g
Mass of oxygen = 4.00 g
Which is limiting reactant = ?
Balance chemical equation:
4NH₃ + 3O₂ → 2N₂ + 6H₂O
Number of moles of ammonia:
Number of moles = mass/molar mass
Number of moles = 2.00 g/ 17 g/mol
Number of moles = 0.12 mol
Number of moles of oxygen:
Number of moles = mass/molar mass
Number of moles = 4.00 g/ 32 g/mol
Number of moles = 0.125 mol
Now we will compare the moles of ammonia and oxygen with water and nitrogen.
NH₃ : N₂
4 : 2
0.12 : 2/4×0.12 = 0.06
NH₃ : H₂O
4 : 6
0.12 : 6/4×0.12 = 0.18
O₂ : N₂
3 : 2
0.125 : 2/3×0.125 = 0.08
O₂ : H₂O
3 : 6
0.125 : 6/3×0.125 = 0.25
The number of moles of water and nitrogen formed by ammonia are less thus ammonia will be limiting reactant.
Amount of oxygen left:
NH₃ : O₂
4 : 3
0.12 : 3/4×0.12= 0.09
Amount of oxygen react = 0.09 mol
Amount of oxygen left = 0.125 - 0.09 = 0.035 mol