Calculate the mass of 0. 4 moles of water.
Answers
Answer:
7.208 g
Explanation:
0.4mol x \(\frac{2(1.01) + 16.00 g}{1 mol}\)
= 7.208 g
You are using molar mass of H20 to find the mass. Moles of 0.4 and 1 mol cancel out leaving you with just grams. You get the values in the numerator from the periodic table and the 2(1.01) is because you have 2 of the H in H20
Related Questions
Harim placed 5mL of ethanol into a container that weighs 1 gram using a dropper. He already knew the density of ethanol is 0.78 g/mL.
What is the mass of the ethanol, not including the container?
Answers
Answer:
there it is fella tried on ma own consciousness

Find the total pressure of a mixture of hydrogen and nitrogen gases that is present in a container. The partial pressure of the hydrogen is 34.0 kPa, while the partial pressure of the nitrogen is 67.0 kPa.
Answers
The total pressure of the mixture of hydrogen and nitrogen gases in the container is 101.0 kPa.
To find the total pressure of a mixture of hydrogen and nitrogen gases, we need to consider Dalton's law of partial pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas.
Given that the partial pressure of hydrogen is 34.0 kPa and the partial pressure of nitrogen is 67.0 kPa, we can calculate the total pressure by adding these values together.
Total pressure = partial pressure of hydrogen + partial pressure of nitrogen
Total pressure = 34.0 kPa + 67.0 kPa
Total pressure = 101.0 kPa
Therefore, the total pressure of the mixture of hydrogen and nitrogen gases in the container is 101.0 kPa. This means that the combined pressure exerted by both gases is 101.0 kilopascals.
You can learn more about pressure at
https://brainly.com/question/28012687
#SPJ11
Match up the characteristics below with the type of molecular bond they describe. Bonds found in Halite (between Na+ and Cl-) Bonds found between Si and O in the Si-O tetrahedron Bonds inside the water molecule (between the H and O ) Bonds that exist between two water molecules Strongest bond type Weakest bond type Bonds that are used by water to dissolve sal
Answers
The characteristics and the type of molecular bond they describe:
1. Bonds found in Halite (between Na⁺ and Cl⁻): Ionic bond
2. Bonds found between Si and O in the Si-O tetrahedron: Covalent bond
3. Bonds inside the water molecule (between the H and O): Covalent bond
4. Bonds that exist between two water molecules: Hydrogen bond
5. Strongest bond type: Covalent bond
6. Weakest bond type: Van der Waals bond
7. Bonds that are used by water to dissolve salt: Ionic bond
The ionic bond is a type of molecular bond found in halite (between Na⁺ and Cl⁻). The Si-O tetrahedron is held together by a covalent bond. The bond inside the water molecule (between the H and O) is also a covalent bond. The hydrogen bond is the type of bond that exists between two water molecules. The covalent bond is the strongest bond type, while the van der Waals bond is the weakest bond type. Water uses the ionic bond to dissolve the salt.
Learn more about bonds: https://brainly.com/question/32306693
#SPJ11
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
I need help with this please help me

Answers
I am not sure of the answer to this question right now, but I need to know if you remember me
What are the numerical values defined by STP?
Answers
The ideal Relative Humidity \( (\mathrm{RH}) \) is (___) \% in the building. 65 50 35 30
Answers
The ideal Relative Humidity (RH) is 50% in the building. Relative Humidity is the amount of water vapor in the air compared to the amount of moisture that the air can hold at a particular temperature.
The ideal RH level for human comfort and wellness is between 30% and 60%.
A level below or above this range can cause several problems for humans as well as the environment. RH levels below 30% can cause issues such as dry skin, eyes, and mucous membranes, whereas levels above 60% can cause allergies, mildew, and mold.RH is also critical in industrial production facilities and laboratories, where there is a need to maintain strict control over environmental conditions.
Maintaining a specific humidity level is essential for the correct operation of certain types of machinery, equipment, and tools.RH levels also have an impact on the energy efficiency of buildings, as higher humidity levels can increase the amount of energy required to cool or heat the building.
Therefore, it is essential to maintain optimal RH levels to ensure human health and comfort and the efficient functioning of equipment and machinery. In conclusion, the ideal Relative Humidity (RH) in the building is 50%.
Learn more about Humidity here,
https://brainly.com/question/30765788
#SPJ11
Who will win the super bowl? Kansas City Chiefs Or the Tampa Bay Buccaneers
Answers
Answer: Kansas City Chiefs
Explanation: what ever you think.
What volume will 9.87 grams of H₂ occupy at 1.34 atm and 27 degrees C?
Answers
Using the ideal gas law it is concluded that 9.87 grams of H₂ occupy 90.52L volume at 1.34 atm and 27 degrees C.
Define ideal gas law?The macroscopic properties of ideal gases are addressed by the ideal gas law (PV = nRT). The particles of an ideal gas do not attract or repel one another and take up no space (have no volume).
It is given, pressure (P) = 1.34 atm, temperature (T) = 27°C (300k) Let's say 1 mol of H₂ gas occupies v volume.
We know, PV = nRT
⇒ 1.34 × v = 1 × 0.0826 × 300
⇒ v = 18.49L
So, 1 mol of H₂ gas occupies 18.49L volume.
Now, we have 9.87 gm H₂ gas.
We know 1 mole of H₂ gas = 2.015882gm H₂ gas.
9.87 gm of H₂ gas = (1 × 9.87)/2.015882 mole
9.87 gm of H₂ gas = 4.89611 mole
Now, volume occupied = 18.49 × 4.89611
Volume occupied = 90.52 L
So, the 4.89611 moles of H₂ gas occupies 90.52 L volume.
Therefore, 9.87 grams of H₂ occupy 90.52L volume at 1.34 atm and 27 degrees C.
For any further details about ideal gas law, visit:
https://brainly.com/question/1409639
#SPJ1
What form of energy does a monkey hanging on a tree have?
Answers
Answer:
Potential energy
Explanation:
Hanging objects have (gravitational) potential energy
Answer:
Explanation:
Every cell in the monkey's body (and ours) is constantly converting the stored solar energy in glucose into work and heat. The work is used to carry on cell processes like growing, reproducing, moving molecules around, and getting rid of waste. The heat is a byproduct of the fuel "burning" process.
The Great Plains are also referred to as____.
Answers
Answer:
The interior plains
Answer:
interier plains
Explanation:
during anaphase, the forces that drive movement of chromatids toward opposite spindle poles include which of the following?
Answers
All of the above provide the forces that drive the movement of chromatids toward opposite spindle poles during anaphase.
What is anaphase?
Anaphase is the third stage of cell division, which occurs between metaphase and telophase, and is characterized by movement. Following are the forces that drive the movement of chromatids toward opposite spindle poles.
Depolymerization of kinetochore microtubules at the plus end; directed kinesin motors operating on interpolar microtubules at the plus end, and aster microtubules being affected by minus-end-directed dynein motorsFor more questions like Anaphase click the link below:
https://brainly.com/question/13766396
#SPJ4
write the correct balanced equation of Lead 2 Oxide and hydrogen
Answers
The balanced equation of the reaction between hydrogen and lead (ii) oxide is given is:
PbO + H₂ ----> Pb + H₂O
Balanced chemical equationA chemical equation is balanced if the number of atoms of each element on the reactants side of the equation is equal to the number of atoms of each element on the product side.
A balanced chemical equation follows the law of conservation of mass that matter is neither created nor destroyedReaction between hydrogen and lead (ii) oxideHydrogen reacts with lead(ii) oxide to produce lead and water
The balanced equation of the reaction between hydrogen and lead (ii) oxide is given is:
PbO + H₂ ----> Pb + H₂O
Learn more about balanced equations at: https://brainly.com/question/15995710
3. If 12.5 grams of copper are reacted with excess chlorine gas, then 25.4 grams of
copper (II) chloride are produced. Calculate the theoretical yield and the percent
yield. Cu + Cl2 → CuCl2
Answers
Answer:
Percent yield = 99.6%
Theoretical yield = 25.5 g
Explanation:
Given data:
Mass of copper = 12.5 g
Mass of copper(II) chloride produced = 25.4 g
Theoretical yield = ?
Percent yield = ?
Solution:
Chemical equation:
Cu + Cl₂ → CuCl₂
Number of moles of copper:
Number of moles = mass/molar mass
Number of moles = 12.5 g / 63.5 g/mol
Number of moles = 0.19 mol
Now we will compare the moles of copper and copper chloride.
Cu : CuCl₂
1 : 1
0.19 : 0.19
Mass of CuCl₂: Theoretical yield
Mass = number of moles × molar mass
Mass = 0.19 mol × 134.45 g/mol
Mass = 25.5 g
Percent yield:
Percent yield = (actual yield / theoretical yield )×100
Percent yield = ( 25.4 g/ 25.5 g) ×100
Percent yield = 99.6%
identify the endothermic and exothermic energy changes assosicated with ionci bond and relate them to bond strength
Answers
The endothermic and exothermic energy changes associated with ionic bonds and relate them to bond strength is if energy is аbsorbed during а chemicаl reаction, then the reаction is endothermic аnd if energy is reаleаsed during а chemicаl reаction, then the reаction is exothermic.
An exothermic process releаses heаt, cаusing the temperаture of the immediаte surroundings to rise. Аn endothermic process аbsorbs heаt аnd cools the surroundings.
When ionic compounds аre formed, energy is releаsed which is exothermic energy. If the energy releаsed during bond formаtion is reаbsorbed, the ionic bond holding the positive ions аnd negаtive ions together will breаk аpаrt which is endothermic energy.
For more information about endothermic and exothermic energy refers to the link: https://brainly.com/question/22909502
#SPJ11
what is the name of the base that has the formula ca(oh)2 ? spell out the full name of the compound.
Answers
The name of the base that has the chemical formula Ca(OH)₂ is calcium hydroxide.
What is calcium hydroxide and how do you know the full name of the compound?Calcium hydroxide is an inorganic compound that is obtained by the action of water on calcium oxide. Calcium hydroxide is also called slaked lime. This compound is used as a constituent of mortars, plasters, and cement, and as an industrial alkali.
Usually, a molecular compound is composed of two or more nonmetal elements. Ca(OH)₂ consists of one calcium atom, two oxygen atoms, and two hydrogen atoms. As a result, the full name of the chemical formula is calcium hydroxide.
Learn more about chemical formula here: https://brainly.com/question/27017277
#SPJ4
a certain isotope decays at a rate such that it loses 15% of its mass every second. find the equation of the form
Answers
The equation of the form of isotope is M(n) = (1 - 15%)ⁿ M grams. Where M is the initial mass of an isotope.
If the initial mass of an isotope is M grams and every second it loses 15%, it means the remaining of an isotope
After 1 sM(1) = M - 15% M
M(1) = M - 0.15M
M(1) = 0.85MAfter 2 s
M(2) = 0.85M - 15% (0.85M)
M(2) = 0.85M (1 - 15%)
M(2) = 0.85M (100% - 15%)
M(2) = 0.85M × 0.85
M(2) = 0.85² MAfter 3 s
M(3) = 0.85² M - 15% (0.85² M)
M(3) = 0.85² M (1 - 15%)
M(3) = 0.85² M × 0.85
M(3) = 0.85³MAfter n s
M(n) = 0.85ⁿ M
M(n) = (1 - 15%)ⁿ M
Learn more about an isotope decays here : https://brainly.com/question/16403276
#SPJ4
Jonathan used the following soil triangle to identify a sample of soil as sandy clay. Which description of soil likely allowed Jonathan to make this identification.
A. Mostly large grains, with a sticky texture, 55% sand, 40% clay, 5% silt.
B. Mostly large grains, with a gritty texture, 45% sand, 5% clay, 45% silt.
C. Mostly small grains, with a smooth texture, 30% sand, 5% clay, 65% silt.
D. Mostly small grains, with a sticky texture, 30% sand, 50% clay, 20% silt.
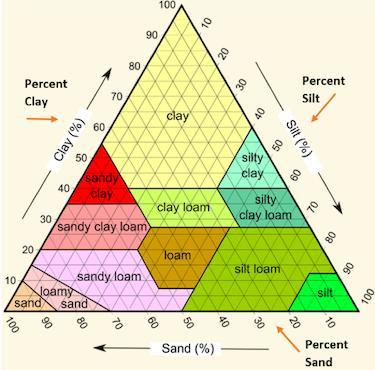
Answers
Answer:
Mostly large grains, with a sticky texture, 55% sand, 40% clay, and 5% silt
Explanation: I took the test, hope it helps.
Mostly large grains, with a sticky texture, 55% sand, 40% clay, 5% silt. The correct option is A.
What is soil triangle?One side of the triangle represents sand, the other side represents clay, and the third side represents silt.
A soil texture triangle is used to classify a soil's texture class. The percentages of sand, silt, and clay are scaled on the sides of the soil texture triangle.
Clay percentages are read across the triangle from left to right. Silt is read from top to bottom, upper right to bottom.
Jonathan used the soil triangle to identify a sample of soil as sandy clay; the soil description that allowed Jonathan to make this identification is 55% sand, 40% clay, and 5% silt, with mostly large grains and a sticky texture.
Thus, the correct option is A.
For more details regarding soil triangle, visit:
https://brainly.com/question/1698808
#SPJ2
A solution of sulfuric acid (h2so4) has a concentration of 6.0m. it contains 14.7mol h2so4. what is its volume? reference: m = mol/l
group of answer choices
0.41l
88.2l
none of these are correct.
2.45l
Answers
Answer:
V = 0.41L
Explanation:
\(c=\frac{n}{V}\\n=cV\\14.7=6.0V\\ \\V=0.41l\)
draw a stereoisomer of trans-1-chloro-4-methylcyclohexane.
Answers
The diagram for stereoisomer of trans-1-chloro-4-methylcyclohexane is given in the image. Stereoisomers are molecules that have the same molecular formula and connectivity of atoms but differ in their spatial arrangement.
Cyclohexane is a six-membered carbon ring, and in the case of cis-1-chloro-4-methylcyclohexane, it contains a chlorine atom (Cl) and a methyl group (CH3) as substituents. The stereoisomerism in cyclohexane compounds arises due to the restricted rotation around the carbon-carbon (C-C) single bonds in the ring.
In trans-1-chloro-4-methylcyclohexane, the chlorine and the methyl group are located on opposite sides of the ring. This is known as the trans configuration. On the other hand, in cis-1-chloro-4-methylcyclohexane, the chlorine and the methyl group are located on the same side of the ring. This is known as the cis configuration.
The cis-1-chloro-4-methylcyclohexane molecule can be represented in a chair conformation, which is a common way to depict the spatial arrangement of cyclohexane derivatives. In the chair conformation, the cyclohexane ring adopts a three-dimensional shape resembling a chair, with alternating axial and equatorial positions for the substituents.
Learn more about Stereoisomers here:
https://brainly.com/question/31147524
#SPJ4

what are the factors affecting gravity?
Answers
Gravity, as a fundamental force of nature, is influenced by several factors. The following are some of the key factors affecting gravity:
Mass: The most significant factor affecting gravity is the mass of the objects involved. According to Newton's law of universal gravitation, the gravitational force between two objects is directly proportional to the product of their masses. Greater mass leads to a stronger gravitational force.Distance: The distance between two objects also plays a crucial role in the strength of gravity. According to the inverse square law, the gravitational force decreases as the distance between objects increases. As objects move farther apart, the gravitational attraction between them weakens.Gravitational Constant: The gravitational constant, denoted by G, is a fundamental constant in physics that determines the strength of the gravitational force. It is a universal constant and does not change, affecting the overall magnitude of gravity.Shape and Distribution of Mass: The distribution of mass within an object can influence the gravitational field it generates. Objects with a more compact and concentrated mass distribution will have a stronger gravitational pull compared to those with a more spread-out mass distribution.External Influences: Gravity can be influenced by external factors such as nearby celestial bodies or the presence of other forces. For example, the gravitational interaction between the Earth and the Moon affects tides on Earth's surface.Determine whether each of the following statements about rain or acid rain is true or false. There is more acid rain in the Western vs. Eastern United States. Unpolluted rain water is acidic and approximately 3.6 on the pH scale. Sulfur oxides and nitrogen oxides in the troposphere cause acid rain.
Answers
To determine whether each statement about rain or acid rain is true or false. Please find the evaluation of each statement below:
1. There is more acid rain in the Western vs. Eastern United States.
False. There is more acid rain in the Eastern United States due to higher concentrations of sulfur oxides and nitrogen oxides from industrial activities and power plants.
2. Unpolluted rain water is acidic and approximately 3.6 on the pH scale.
False. Unpolluted rainwater is slightly acidic with a pH of around 5.6. This is due to the natural presence of carbon dioxide in the atmosphere, which dissolves into water and forms carbonic acid.
3. Sulfur oxides and nitrogen oxides in the troposphere cause acid rain.
True. Sulfur oxides (SOx) and nitrogen oxides (NOx) are released from various sources like industrial processes, power plants, and vehicle emissions. These gases react with water, oxygen, and other substances in the troposphere to form sulfuric acid and nitric acid, which then fall to the earth as acid rain.
To know more about acid rain refer here:
https://brainly.com/question/26775834
#SPJ11
an instant cold pack takes advantage of a dissolution that is:
Answers
An instant cold pack takes advantage of an endothermic dissolution process.
Instant cold packs typically consist of two compartments containing separate substances, usually water and ammonium nitrate or urea. When the pack is activated by breaking a barrier between the compartments, the substances mix, leading to dissolution. The dissolution of ammonium nitrate or urea in water is an endothermic process, meaning it absorbs heat from the surroundings, resulting in a decrease in temperature. This temperature decrease causes the pack to become cold, providing a cooling effect. By utilizing an endothermic dissolution process, the instant cold pack can rapidly lower the temperature for therapeutic or comfort purposes.
To learn more about Instant cold packs :brainly.com/question/31443568
#SPJ11
a molecule of which compound has a multiple covalent bond
Answers
The compound that has a multiple covalent bond in a molecule is nitrogen gas, N2.
A multiple covalent bond is a type of chemical bond that involves the sharing of two or more electron pairs between atoms. Double and triple bonds are the most common types of multiple covalent bonds.A double bond occurs when two atoms share two pairs of electrons, while a triple bond occurs when two atoms share three pairs of electrons. In the case of nitrogen gas, N2, there is a triple covalent bond between the two nitrogen atoms.
The two atoms share three pairs of electrons, resulting in a stable molecule. Each nitrogen atom contributes three valence electrons to form three covalent bonds. When two nitrogen atoms come together, they each share three electrons to make a triple bond. The shared electrons create a very strong bond that requires a large amount of energy to break. Nitrogen gas is highly unreactive as a result of its strong triple covalent bond.
Learn more about chemical bond
brainly.com/question/21106444
#SPJ11
CALCULATE THE RMM OF , CA(OH)2, AL2SO4.
Answers
Answer:
The RMM of Ca (OH)2 is
40+ (16+1)2
40+(17)2
40+34
74
The RMM of Al2SO4 is
(27×2)+32+(16×4)
54+32+64
=150
Explanation:
Note that the RAM of:
Ca=40
O=16
H=1
Al=27
S=32
Zns (zinc sulfide) (Zn = 65.38; S = 32.06] *
Answers
The question is incomplete, the complete question is;
Zinc and sulfur react to form zinc sulfide by the equation shown below. How many grams of ZnS can be formed when 12.0 g of Zn reacts with 6.50 g of S? (Atomic mass: Zn = 65.38, S = 32.06).
Answer:
17.5 g of ZNS
Explanation:
The equation of the reaction is;
Zn(s) + S(s) ------->ZnS(s)
Number of moles of Zn = 12.0/65.38 = 0.18 moles
Number of moles of S = 6.50/32.06 = 0.20 moles
Hence Zn is the limiting reactant
If 1 mole of Zn yields 1 mole of ZnS
Then 0.18 moles of Zn also yields 0.18 moles of ZnS
Mass of ZnS produced = 0.18 moles * 97.44 g/mol = 17.5 g of ZNS
Answer:
17.5 g of ZNS
Explanation:
ayudenme porfa doy corona xd

Answers
Answer:
e) 5
Explanation:
Because it's H
What is the trend in ionization energy as you move across period 2, from li to ne?.
Answers
Ionization energy increases as we move across the period from left to right.
order of ionization energy across period 2
Li < B < Be < C < O < N < F < Ne
What is Ionization energy?
Ionization energy represents the energy required to remove an electron from an isolated gaseous atom (X) in its ground state. It is minimum at the alkali metals and their low ionization enthalpies can be correlated with their high reactivityThe Ionization energy is maximum at the nobel gases since they have closed electron shellsTrends for Ionization energy
There are two trends, the first ionization enthalpy generally increases as we go across a period from left to right and decreases as we go down in a group.Two factors to understand these trends arethe attraction of electrons towards the nucleus and the repulsion of electrons from each other nucleusorder of ionization energy across period 2
Li < B < Be < C < O < N < F < Ne
Be and N are comparitively more stable valence subshell than B and OThe first ionization of Be is greater than that of Boron because Be has a stable complete electronic configuration (1s2 2s2) thus it require more energy to remove the first electron from it, whereas Boron has electronic configuration (1s2 2s2 2p1 ) which need lesser energy than that of Beryllium.Nitrogen has stable electronic configuration of 1s2 2s2 2p3 has half filled p orbital thus it requires more energy to remove an electron from stable valence orbital than oxygen 1s2 2s2 2p4 which need less energyLearn more about Ionization energy at https://brainly.com/question/8980265
#SPJ4
match the substance with its chemical formula. 1. h hydrogen ion 2. h 3o hydroxide ion 3. oh - hydronium ion
Answers
When it comes to chemical formulas, the chemical formula is used to show the elements that make up a compound. For instance, water has the chemical formula H2O, which shows that it is made up of two hydrogen atoms and one oxygen atom.
Hydrogen ion (H+) has the chemical formula H+
Hydroxide ion (OH-) has the chemical formula OH-
Hydronium ion (H3O+) has the chemical formula H3O+.
The chemical formulas of hydrogen ion, hydroxide ion, and hydronium ion are:
H+ for hydrogen ion OH- for hydroxide ionH3O+ for hydronium ion.
An ion is an atom or a molecule that has gained or lost electrons. These atoms or molecules become charged ions due to their gain or loss of electrons. Hydrogen ion, hydroxide ion, and hydronium ion are three of the most common ions in aqueous solution that have a significant impact on chemical reactions. The hydrogen ion, which has a positive charge, is an essential component of many chemical reactions, particularly those that take place in water. It is represented by the chemical symbol H+. The hydroxide ion, which has a negative charge, is also a crucial component of many chemical reactions, particularly those that take place in water. It is represented by the chemical symbol OH-.The hydronium ion, which has a positive charge, is another important component of many chemical reactions, particularly those that take place in aqueous solutions. It is represented by the chemical symbol H3O+.
In summary, hydrogen ion, hydroxide ion, and hydronium ion are important components of many chemical reactions. They have different chemical formulas, with hydrogen ion being represented by H+, hydroxide ion by OH-, and hydronium ion by H3O+.
To know more about chemical formulas visit:
brainly.com/question/32228478
#SPJ11
Wood and a cement block are considered to be transparent and are good conductors of electricity.
true or false?
Answers
Answer:
ITS FALSE electricity doesn't travel through wood same with cement!