Calculate the final ph of a solution made by the addition of 10 ml of a 0.5 m naoh solution to 500 ml of a 0.4 m ha originally at ph = 5.0 (pka = 5.0) neglect the volume change.
Answers
The final pH of a solution is 5.02 made by the addition of 10 ml of a 0.5 m NaOH solution to 500 ml.
What is pH?
The primary pH standard values are calculated using a concentration cell with transference, by measuring the potential difference between a hydrogen electrode and a standard electrode such as the silver chloride electrode. The pH scale is traceable to a set of standard solutions whose pH is established by international agreement. With the use of a glass electrode, a pH meter, or a color-changing indicator, the pH of aqueous solutions can be determined. In chemistry, agronomy, medicine, water treatment, and many other fields, pH measurements are crucial.
A scale called pH is used in chemistry to describe how basic or acidic an aqueous solution is. Lower pH values are recorded for acidic solutions (solutions with higher H+ ion concentrations) than for basic or alkaline solutions.
To learn more about pH from the given link:
https://brainly.com/question/172153
#SPJ4
Related Questions
A/an
is larger than the neutral atom.
Answers
Answer:
Anions are larger than their corresponding neutral atoms
why is it important to have portable incubator that can be used without constant electricity?
Answers
It is crucial to maintain a constant temperature because the incubator will work with living things. Additionally, these samples are quite delicate.
Why is high temperature tolerance important?Designing materials for use in engineering applications at high temperatures requires consideration of thermal stability. The ability of an alloy to maintain ductility and toughness during prolonged temperature exposure can be referred to as this property.
How does stability differ depending on temperature?Several growth restrictions are present for organisms developing at low temperatures: nucleic acid structures become much more solid, membrane fluidity diminishes, enzyme reaction rates slow down, affinity of transport and uptake systems declines.
To know more about stable temperature visit:
https://brainly.com/question/6655856
#SPJ1
How much energy would 10g of water at 150°C need to gain or lose to reach 50°C?
Round to the Third Non-Zero
No commas
Only place a sign if a value is negative and not positive.
1. What is the energy in the solid phase
2. What is the energy in the melting/fusion transition
3. What is the energy in the liquid phase
4. What is the energy in the vaporization/condensation transition
5. What is the energy in the gas phase
6. What is the total energy
Answers
The 10g of water at 150°C would need to lose 4184 J of energy to reach 50°C.
How we calculated energy?To calculate the energy needed for 10g of water at 150°C to reach 50°C, we need to use the specific heat capacity of water and the formula:
Q = mcΔT
where Q is the energy needed, m is the mass of water, c is the specific heat capacity of water, and ΔT is the change in temperature.
1. Since the water is not in the solid phase, there is no energy in the solid phase.
2. Since the water is not undergoing a melting/fusion transition, there is no energy in the melting/fusion transition.
3. The energy in the liquid phase is given by:
Q = mcΔT = 10g x 4.184 J/g°C x (50°C - 150°C)
= -4184 J (negative sign indicates a loss of energy)
4. Since the water is not undergoing a vaporization/condensation transition, there is no energy in the vaporization/condensation transition.
5. Since the water is not in the gas phase, there is no energy in the gas phase.
6. The total energy needed is the sum of the energies in each phase, which is -4184 J
Learn more about energy
brainly.com/question/1932868
#SPJ11
Which of the following is not a renewable source of energy?
1.) geothermal
2.) coal
3.) solar
4.) wind
Answers
2. Coal
It is non renewable energy source.
Answer: the answer would be coal
Explanation: hoped this helped!
(Blank) used for lightning protection systems are manufactured from copper-clad steel, solid copper, and stainless steel materials
Answers
Lightning protection systems utilize materials, including copper-clad steel, solid copper, and stainless steel, chosen for their specific properties and characteristics making them lightning protection apps.
Lightning protection systems are designed to safeguard structures and people from the destructive forces of lightning strikes. The materials used in these systems play a crucial role in dissipating and redirecting the electrical energy of lightning safely. Copper-clad steel is a popular choice for lightning rods and conductors due to its combination of strength and conductivity.
The steel core provides mechanical strength, while the copper coating ensures excellent electrical conductivity. Solid copper is another common material used in lightning protection systems. Copper has exceptional electrical conductivity and is highly resistant to corrosion, making it an ideal choice for applications where longevity and conductivity are vital. Additionally, stainless steel, known for its durability and corrosion resistance, is utilized in lightning protection systems, particularly for grounding components and structural connections.
By using these materials strategically within lightning protection systems, the risk of damage from lightning strikes can be minimized by providing effective paths for lightning current to safely follow, reducing the potential for destructive electrical surges.
To learn more about stainless steel click here: brainly.com/question/30757610
#SPJ11
Give the formula for the compound formed when magnesium oxide reacts with water. Express your answer as a chemical formula.
Answers
The compound formed when magnesium oxide reacts with water is magnesium hydroxide (Mg(OH)₂).
Magnesium oxide (MgO) is an ionic compound consisting of magnesium cations (Mg²⁺) and oxide anions (O²⁻). When it reacts with water (H₂O), the oxygen atom in water attracts the magnesium cation, leading to the formation of magnesium hydroxide.
The reaction can be represented as follows:
MgO + H₂O → Mg(OH)₂
In the resulting compound, magnesium hydroxide, there are two hydroxide ions (OH-) for every magnesium ion (Mg²⁺). The chemical formula Mg(OH)₂ represents this ratio.
Magnesium hydroxide is an alkaline compound and is commonly used as an antacid to treat heartburn and indigestion. It is also used in various industrial applications.
To know more about magnesium hydroxide, refer here:
https://brainly.com/question/12891796#
#SPJ11
Now suppose a reaction vessel is filled with 0.0406 atm of nitrogen (N_2) and 5.97 atm of ammonia (NH_3) at 1126. Degree C. Answer the following question this system: Under these conditions, will the pressure of N_2 tend to rise or fall? rise fall Is it possible to reverse this tendency by adding H_2? In other words, if you said the pressure of N_2 will tend to rise, can that be changed to a tendency to fall adding H_2? Similarly, if you said the pressure of N_2 will tend to fall, can that be changed to a tendency to rise by adding H_2? Yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of H_2 needed to reverse it. Round your answer to 2 significant digits. atm
Answers
The pressure of \(N_{2}\) will rise under the given conditions. And, Yes, it is possible to reverse this tendency by adding \(H_{2}\). The minimum pressure of H2 required to reverse the tendency is 0.01 atm.
The reaction involved is: \(N_{2}\)(g) + 3\(H_{2}\)(g) ⇌ 2\(NH_{3}\)(g) Hence, when \(H_{2}\) is added to the above system, the \(N_{2}\) and \(H_{2}\) will react to produce \(NH_{3}\). This reaction will reduce the amount of \(N_{2}\) present in the system, causing the pressure of \(N_{2}\) to decrease. Therefore, by adding \(H_{2}\) , we can change the tendency of \(N_{2}\) pressure from rise to fall.To calculate the minimum pressure of \(H_{2}\) required to reverse the tendency, we have to use the equilibrium constant, Kp. The expression for Kp for the above reaction is: Kp =( \(NH_{3}\)) / p(\(N_{2}\)) p3( \(H_{2}\) )
At equilibrium, Kp = 1.7 × 104 at 1126 °C.Now, we will solve for the minimum pressure of \(H_{2}\) needed to reverse the tendency. Let's assume that the pressure of \(N_{2}\) has increased by x atm. Therefore, the new pressure of \(N_{2}\) will be (0.0406 + x) atm. At equilibrium, we have:
p2(\(NH_{3}\) ) / p(\(N_{2}\)) p3( \(H_{2}\) ) = 1.7 × 104
On substituting the given values and simplifying, we get:
p2(\(NH_{3}\)) / p(N2) = 6.39 × 10-5
Now, p2(\(NH_{3}\)) = 5.97 atm, and p(\(N_{2}\)) = (0.0406 + x) atm.
On substituting these values, we get:5.97 / (0.0406 + x) = 6.39 × 10-5
Solving for x, we get:x = 0.00579 atm ≈ 0.01 atm (rounded to 2 significant digits)Therefore, the minimum pressure of \(H_{2}\) required to reverse the tendency is 0.01 atm.
More on equilibrium: https://brainly.com/question/30188799
#SPJ11
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
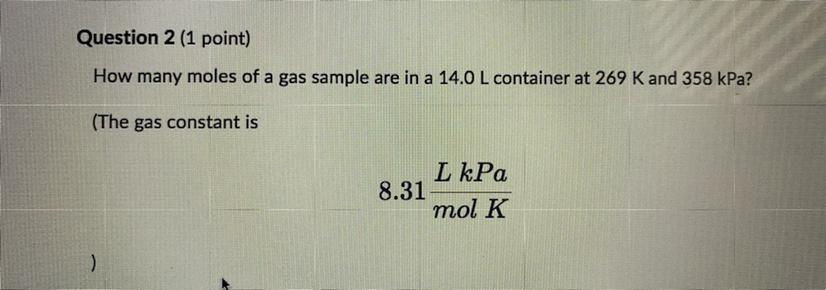
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
What would the mass be of a single atom of copper?
Answers
Answer:
1
atom of
Cu
has a mass of
1.055
×
10
−
22
g
.
a solution is 0.20 m ba(oh)2(aq). how many moles of hcl(aq) must be added to neutralize 100.0 ml of the solution?
Answers
A solution is 0.20 m ba(oh)2(aq). 0.040 moles of hcl(aq) must be added to neutralize 100.0 ml of the solution
number of moles of Ba(OH)2 = molarity * volume of solution in L
number of moles of Ba(OH)2 = 0.20 * 0.10 L = 0.020 mole
Ba(OH)2 + 2H+ ----> Ba^2+ + 2H2O
from the balanced equation we can say that
1 mole of Ba(OH)2 requires 2 mole of H+ so
0.020 mole of Ba(OH)2 will require
= 0.020 mole of Ba(OH)2 *(2 mole of H+ / 1 mole of Ba(OH)2)
= 0.040 mole of H+
Therefore, the number of moles of H+ required are 0.040 mol
learn more about Ba(OH)2 here:
https://brainly.com/question/14958132
#SPJ4
When 0.1 moles of atoms of an element reacts with chlorine there is an increase in mass of 7.1g
Answers
Answer:
Carbon
Explanation:
9 When 0.1 mol of atoms of an element reacts with chlorine, there is an increase in mass of 7.1 g. The element could be carbon.
A set of solubility data is given below.
What is the mass of the dry solute
recovered?
Sample
2
Temperature
(°C)
30.1
Boat Mass
(8)
0.730
Boat +
Solution (g)
0.929
Boat + Dry
(g)
0.816

Answers
Answer:
0.086
Explanation:
got it on acellus
The mass of the dry solute recovered from the given data is 0.086 g. Option C
To determine the mass of the dry solute recovered, we need to subtract the mass of the boat from the mass of the boat with the dry solute.
Given the data provided:
Boat Mass: 0.730 g
Boat + Solution: 0.929 g
Boat + Dry: 0.816 g
To find the mass of the dry solute, we subtract the boat mass from the boat + dry mass:
Mass of Dry Solute = (Boat + Dry) - (Boat Mass)
Mass of Dry Solute = 0.816 g - 0.730 g
Mass of Dry Solute = 0.086 g
Therefore, the correct answer is c) 0.086 g.
The mass of the dry solute recovered from the given data is 0.086 g. It is important to note that the mass of the dry solute is obtained by subtracting the mass of the boat from the mass of the boat with the dry solute, as the boat mass represents the weight of the empty boat or container used in the experiment.
For more such questions on solute visit:
https://brainly.com/question/25326161
#SPJ8
When chlorine gas comes into contact with magnesium metal at high temperatures, solid magnesium chloride is created. Classify this reaction.
Hint: It may help to write the balanced equation.
a) double replacement
b)synthesis
c) decomposition
d) single replacement
Answers
The balanced equation for this reaction is: 2Mg + \(Cl_{2}\) → \(2MgCl_{2}\).
Based on this, the classification of the reaction is (d) single replacement.
The given chemical equation is:
Mg(s) + \(Cl_{2}\)(g) → \(MgCl_{2}\)(s)
In this reaction, magnesium metal (Mg) reacts with chlorine gas (\(Cl_{2}\)) to form solid magnesium chloride (\(MgCl_{2}\)).
During the reaction, the magnesium atoms lose electrons to form magnesium ions with a +2 charge, while the chlorine molecules gain electrons to form chloride ions with a -1 charge. These ions then combine to form the ionic compound, magnesium chloride.
Based on the type of reaction, this can be classified as a synthesis reaction, where two or more substances combine to form a single, more complex substance.
Therefore, option (b) - synthesis is the correct classification for the given chemical reaction.
To learn more about balanced equation click on the given link brainly.com/question/11904811
#SPJ1
Part D
Calculate the following for test tube 1 and for test tube 2, and record the results in the table:
the number of moles of copper(II) sulfate used (Use 159.60 grams/mole as the molar mass of copper(II) sulfate.)
the heat absorbed by the water, in joules (Use Q = mCΔT, where 10.0 milliliters of water has a mass of 10.0 grams. Use 4.186 joules/gram degree Celsius as water’s specific heat capacity.)
the change in internal energy of the copper(II) sulfate (Assume that the energy released by the copper(II) sulfate is absorbed by the water.)
the reaction enthalpy, in joules/mole



Answers
Learn more about Chemical Name from the given link
brainly.com/question/29594386
#SPJ1
To calculate the following for test tube 1 and test tube 2:
1. The number of moles of copper(II) sulfate used:
Test tube 1: 0.2 g of copper(II) sulfate was used, which is equivalent to 0.001255 moles (0.2 g / 159.60 g/mol).
Test tube 2: 0.4 g of copper(II) sulfate was used, which is equivalent to 0.002510 moles (0.4 g / 159.60 g/mol).
2. The heat absorbed by the water, in joules:
Test tube 1: Q = (10.0 g) x (4.186 J/g°C) x (20.0°C) = 837.2 J
Test tube 2: Q = (10.0 g) x (4.186 J/g°C) x (30.0°C) = 1257.9 J
3. The change in internal energy of the copper(II) sulfate:
Since the energy released by the copper(II) sulfate is absorbed by the water, the change in internal energy of the copper(II) sulfate is equal to the negative of the heat absorbed by the water.
Test tube 1: ΔU = -837.2 J
Test tube 2: ΔU = -1257.9 J
4. The reaction enthalpy, in joules/mole:
The reaction enthalpy can be calculated using the formula ΔH = ΔU + PΔV, where PΔV represents the work done by the system. Assuming that the reaction was carried out at constant pressure (i.e., atmospheric pressure), PΔV can be approximated to zero, and thus the reaction enthalpy is equal to the change in internal energy.
Test tube 1: ΔH = -837.2 J / 0.001255 mol = -666,876 J/mol
Test tube 2: ΔH = -1257.9 J / 0.002510 mol = -500,357 J/mol
Therefore, the results can be recorded in the following table:
| | Moles of CuSO4 used | Heat absorbed by water (J) | Change in internal energy (J) | Reaction enthalpy (J/mol) |
|-----------|---------------------|-----------------------------|---------------------------------|---------------------------|
| Test tube 1 | 0.001255 | 837.2 | -837.2 | -666,876 |
| Test tube 2 | 0.002510 | 1257.9 | -1257.9 | -500,357 |
For more questions on: equivalent
https://brainly.com/question/30196207
#SPJ11
4Al(s) + 30 (9) 2ALO3(s)
Assume that 92 grams of aluminum was reacted at a temperature of 25.0°C
at constant pressure and the reaction temperature rose to 31.41°C. The
specific heat of aluminum is 0.90 J/9°C.
-Calculate AT:
-Calculate change in heat in joules (J). Round to the nearest hundredth.
Answers
I need help please it is my last question and ill give brainy and all the points!

Answers
Answer:
its B
Explanation:
this is pretty obvious since I know about this and did it already.
I Hope this helps!
GOODLUCK!!!!!!!
please help asap i really need help (look at profile)

Answers
Answer:
Explanation:
doing this for point hope you find out tho
A 4M solution means there are
______ moles of ______
per one _____ of _____.
There are _____ ml within _____ Liter.
Q. How many moles of HCL are within 2,000ml of a 0.4M solution of HCL?
Q. How many grams of KCl are within 2L of a 0.4M KCL solution?
Answers
Answer:
Molarity=number of moles÷volume(L)
Given the following reactions:
CaCO3 (s) -> CaO (s) + CO2 (g) H = 178.1
C (s, graphite) + O2 (g) -> CO2 (g) H = -393.5 kJ
The enthalpy of the reation CaCO3 (s) -> CaO (s, graphite) + O2 (g) is _______ kJ
Answers
The enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) is 571.6 kJ.
The enthalpy of the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) can be calculated by summing the enthalpies of the individual reactions involved. The given information provides the enthalpy change for the decomposition of CaCO3 (s) and the combustion of C (s) to form CO2 (g). By combining these reactions, the enthalpy change for the overall reaction can be determined.
The given reactions are:
CaCO3 (s) -> CaO (s) + CO2 (g) (H = 178.1 kJ)
C (s, graphite) + O2 (g) -> CO2 (g) (H = -393.5 kJ)
To calculate the enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g), we need to subtract the enthalpy change of reaction 2 from the enthalpy change of reaction 1. Since the enthalpy change is an extensive property, we can subtract the enthalpies directly:
ΔH = H(reaction 1) - H(reaction 2)
= 178.1 kJ - (-393.5 kJ)
= 178.1 kJ + 393.5 kJ
= 571.6 kJ
Therefore, the enthalpy change for the reaction CaCO3 (s) -> CaO (s, graphite) + O2 (g) is 571.6 kJ.
To learn more about enthalpy click here:
brainly.com/question/32882904
#SPJ11
why is it important to allow the level of solvent to drop below the level of the sand before adding in your sample mixture?.
Answers
It is important to allow the level of solvent to drop below the level of the sand before adding in your sample mixture because if the sample mixture is added before the level of solvent drops below the sand, the sample may mix with the sand causing the resulting solution to be contaminated with sand particles.
Contamination of the solution will impact the accuracy and precision of the analysis.
To prevent this contamination from occurring, the level of solvent should be allowed to drop below the sand before adding the sample mixture. This ensures that the sample will be dissolved in the solvent without interference from sand particles. This practice is especially important in analytical chemistry when trying to obtain precise and accurate results.
To know more about Contamination, refer to the link below:
https://brainly.com/question/32664646#
#SPJ11
In a tank, 27 L He at 25ºC and 101.3 kPa and 12 L O2 at 25ºC and 101.3 kPa are pumped into a tank with a volume of 8.0 L. Calculate the partial pressure of each gas and the total pressure in the tank at 25ºC.
Answers
Total pressure = 4.9 atm
Partial pressure of neon = 3.4 atm
Partial pressure of oxygen = 1.5 atm
What are the mole fractions?We know that the partial pressure could be obtained as the product of the mole fraction and the total pressure thus we have to obtain the total pressure by the use of the partial pressures.
For the number of moles of helium;
P = 101.3 kPa or 0.99 atm
T = 25ºC or 298 K
V = 27 L
n = PV/RT = 0.99 atm * 27 L/0.082 * 298 K = 26.73/24.44 = 1.1 moles
Number of moles of oxygen
P = 101.3 kPa or 0.99 atm
T = 25ºC or 298 K
V = 12 L
n = PV/RT = 0.99 atm * 12 L/0.082 * 298 K =11.88 /24.44 = 0.5 moles
Total number of moles = 1.1 moles + 0.5 moles = 1.6 moles
Total pressure is obtained from;
nRT/V
= 1.6 moles * 0.082 * 298/8
= 4.9 atm
Partial pressure of neon = 1.1 moles/1.6 moles * 4.9 atm = 3.4 atm
Partial pressure of oxygen = 0.5 moles/1.6 moles * 4.9 atm = 1.5 atm
Learn more about partial pressure:https://brainly.com/question/13199169
#SPJ1
in 2017 it was estimated that 47 per cent of Indians worked in the primary sector_mainly in agriculture. why was this sector the least important of the three in terms of outputs
Answers
Factors such as the lack of arable land and the scarcity of water make agriculture less productive in India.
Why does agriculture have the least output in India?India has a large area of degraded and infertile land, which limits the ability of farmers to produce crops. Soil degradation is caused by factors such as deforestation, overgrazing, and soil erosion.
India is facing an increasingly serious water shortage, which affects agriculture in many regions. Lack of access to water for irrigation is a major limiting factor for crop production.
Learn more about Agriculture in India:https://brainly.com/question/30095494
#SPJ1
How many aluminum atoms are in 2.26 g of aluminum?
Answers
Answer:
Aluminum atoms are in 3.78 g of aluminum
02214076x10^23)8.4367659370640769254888x10^22 atoms, therefore there are 8.4367659370640769254888x10^22 atoms of Aluminum(Al) present in 3.78 grams of Aluminum(Al).
Explanation:
formulas for volume
Answers
Shape Formula Variables
Cube V=s3 s is the length of the side.
Right Rectangular Prism V=LWH L is the length, W is the width and H is the height.
Prism or Cylinder V=Ah A is the area of the base, h is the height.

does clapeyron equation, in thermodynamics, help to determine the enthalpy of vaporization hfg at a given temperature from the p, v, t data alone?
Answers
Yes, using only the p, v, and t values, the Clapeyron solution in thermodynamics may be used to compute the energy of vaporization at a particular temperature.
What does the chemistry term "equation" mean?Reaction equation are symbolic depictions of chemical reactions where the reactants and products are stated in relation to the following chemical formulae.
Equation and response are what?What is an equations and a chemical reaction? In a chemical change, bonds between molecules of the reagent are destroyed and new bonds between molecules of the product are established to create a new substance. A chemical formula is nothing more than a statement of fact that represents how reactants generate products.
To know more about Equation visit:
brainly.com/question/28818520
#SPJ4
How many zeros are in the measurement 0.000040200 m are significant?
Answers
Answer:
It’s 3
Explanation:
_________is a chemical or physical agent capable of inducing changes in DNA called mutations
Answers
A mutagen is a chemical or physical agent capable of inducing changes in DNA, leading to mutations.
Mutagens can alter the genetic material by causing changes in the DNA sequence, such as substitutions, deletions, insertions, or rearrangements. These changes can result in the formation of new alleles or the disruption of normal gene function.
Examples of mutagens include certain chemicals, such as certain pesticides, tobacco smoke, and certain chemotherapy drugs. Physical agents like ionizing radiation (e.g., X-rays, gamma rays) and ultraviolet (UV) radiation from the sun or tanning beds can also induce DNA mutations.
It is important to note that not all mutagens are harmful. Some mutations can be beneficial, leading to genetic variation and adaptation in populations, while others may have detrimental effects, such as contributing to the development of diseases like cancer.
To know more about mutagen refer here :
https://brainly.com/question/13960165#
#SPJ11
The overall question is: What will be the approximate energy yield through aerobic metabolism, of a 22-carbon fatty acid? Describe each of the major major reactions involved. Identify the important mo
Answers
The breakdown of a 22-carbon fatty acid through aerobic metabolism via beta-oxidation and the citric acid cycle provides a substantial amount of energy in the form of ATP, allowing cells to perform various vital functions.
The approximate energy yield through aerobic metabolism of a 22-carbon fatty acid involves a series of major reactions within the mitochondria of cells. The process is known as beta-oxidation, and it generates acetyl-CoA molecules that enter the citric acid cycle (also known as the Krebs cycle) to produce ATP.
First, the 22-carbon fatty acid undergoes a series of four reactions in the beta-oxidation pathway. Each cycle of beta-oxidation removes a two-carbon acetyl-CoA molecule from the fatty acid chain, generating one molecule of NADH and one molecule of FADH2 in the process. These high-energy electron carriers will later enter the electron transport chain to produce ATP.
After the beta-oxidation process, the resulting acetyl-CoA molecules enter the citric acid cycle. In this cycle, each acetyl-CoA molecule is oxidized, leading to the production of three molecules of NADH, one molecule of FADH2, and one molecule of GTP (which can be converted to ATP). These electron carriers (NADH and FADH2) will transfer their electrons to the electron transport chain for ATP synthesis.
Finally, the electron transport chain, located in the inner mitochondrial membrane, utilizes the high-energy electrons from NADH and FADH2 to pump protons across the membrane. This establishes an electrochemical gradient that drives ATP synthesis through oxidative phosphorylation. The exact number of ATP molecules generated depends on several factors, but on average, the complete oxidation of a 22-carbon fatty acid yields approximately 129 molecules of ATP.
Overall, the breakdown of a 22-carbon fatty acid through aerobic metabolism via beta-oxidation and the citric acid cycle provides a substantial amount of energy in the form of ATP, allowing cells to perform various vital functions.
Learn more about ATP here,
https://brainly.com/question/897553
#SPJ11
Construct an argument on whether the weight of a pencil would change THREE-DIMENSIONAL THINKING as the pencil falls from 10 m to the ground.
Answers
The gravity of the earth is denoted by the symbol 'g'. Here there is no change in the weight of the pencil as it falls from 10 m to the ground.
What is acceleration due to gravity?The acceleration acquired by an object due to the gravitational force is defined as the acceleration due to gravity. It is generally denoted by the symbol 'g'. Its standard value is 9.8 m/s².
The value of 'g' is not affected by the mass. The factors affecting its value are earth's shape, altitude and depth of the earth's surface. So during the free fall of a pencil there is no air resistance on it.
Thus the weight of the pencil will not change.
To know more about acceleration due to gravity, visit;
https://brainly.com/question/13860566
#SPJ1
4. Which elements have a completely full outermost energy level?
Answers
Answer:
Helium (He), neon (Ne), and argon (Ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. This makes them highly stable as single atoms. Because of their non-reactivity, they are called the inert gases or noble gases.
Explanation:
can i get brainliest