Calculate the energy difference (ΔE) for the transition in Problem 7.24 for 1 mol of H atoms.
Answers
Answer:
The energy difference for the transition in hydrogen atoms for 1 mol is 1.82285 * 10^2 KJ / mol
1 / λ = Rh(1 / n1^2 - 1 / n2^2)
ΔE = Rh (1 / n1^2 - 1 / n2^2)
Rh = 2.18 * 10^-18
ΔE = 2.18 * 10^-18 * (1 / 2^2 - 1 / 3^2)
ΔE = 2.18 * 10^-18 *(1 / 4 - 1 / 9)
ΔE = 3.027 * 10^-19 J
(ΔE)1mol = ΔE * \(N_{A}\)
(ΔE)1mol = 3.027 * 10^-19 * 6.022 * 10^23
(ΔE)1mol = 182285.94 J/mol
(ΔE)1mol = 182.285 KJ / mol
(ΔE)1mol = 1.82285 * 10^2 KJ / mol
The energy difference for the transition in hydrogen atoms for 1 mol is 1.82285 * 10^2 KJ / mol
For more information click on the link below:
brainly.com/question/11176460
# SPJ4
Complete question:
Calculate the energy difference for the electron transition of n = 3 to n = 2 for 1.00 mol of hydrogen atoms.
Related Questions
What element is1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p6
Answers
The electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s² 4f¹⁴ 5d¹⁰ 6p⁶ corresponds to the element Radon (Rn) with atomic number 86.
In the electron configuration, each number and letter combination represents a specific orbital and the number of electrons occupying that orbital. The numbers represent the principal energy levels (or shells) and the letters represent the sublevels (s, p, d, f).
Breaking down the electron configuration;
1s²; This indicates that the first energy level (n=1) has 2 electrons in the 1s orbital.
2s² 2p⁶; The second energy level (n=2) contains 2 electrons in the 2s orbital and 6 electrons in the 2p orbital.
3s² 3p⁶; The third energy level (n=3) has 2 electrons in the 3s orbital and 6 electrons in the 3p orbital.
4s² 3d¹⁰ 4p⁶; The fourth energy level (n=4) contains 2 electrons in the 4s orbital, 10 electrons in the 3d orbital, and 6 electrons in the 4p orbital.
5s² 4d¹⁰ 5p⁶; The fifth energy level (n=5) has 2 electrons in the 5s orbital, 10 electrons in the 4d orbital, and 6 electrons in the 5p orbital.
6s² 4f¹⁴ 5d¹⁰ 6p⁶; The sixth energy level (n=6) contains 2 electrons in the 6s orbital, 14 electrons in the 4f orbital, 10 electrons in the 5d orbital, and 6 electrons in the 6p orbital.
By referring to the periodic table, we can find that the element with this electron configuration is Radon (Rn) with atomic number 86. Radon is a noble gas and is found in the last group (Group 18) of the periodic table.
To know more about electron configuration here
https://brainly.com/question/14283892
#SPJ4
What is the coefficient for water molecules in the balanced version of the following redox reaction? cr2o2−7 c2h4o→c2h4o2 cr3
Answers
The given redox reaction is:
Cr2O7^2- + C2H4O → C2H4O2 + Cr3+
To balance this reaction, we first balance the oxygen atoms by adding H2O on the right side of the equation. The number of H2O molecules added depends on the number of oxygen atoms needed. In this case, we need three O atoms on the right side, so we add three H2O molecules to the right side of the equation:
Cr2O7^2- + C2H4O → C2H4O2 + Cr3+ + 3H2O
Next, we balance the hydrogen atoms by adding H+ ions on the left side of the equation. The number of H+ ions added depends on the number of hydrogen atoms needed. In this case, we need eight H atoms on the left side, so we add eight H+ ions to the left side of the equation:
Cr2O7^2- + C2H4O + 8H+ → C2H4O2 + Cr3+ + 3H2O
Finally, we balance the charge by adding electrons. The number of electrons added depends on the difference in charge on the left and right side of the equation. In this case, the left side has a charge of -2 (from the Cr2O7^2- ion), while the right side has a charge of +3 (from the Cr3+ ion). This means that we need to add 5 electrons to the left side of the equation to balance the charge:
Cr2O7^2- + C2H4O + 8H+ + 5e- → C2H4O2 + Cr3+ + 3H2O
Therefore, the coefficient for water molecules in the balanced version of the given redox reaction is 3.
To know more about redox reaction visit
https://brainly.com/question/28300253?
#SPJ11
Which of the following represents the lattice energy for AlCl3?a)Al(s) + 3/2 Cl2(g) --> AlCl3(s)b)Al(s) --> Al(g)c)3/2 Cl2(g) --> 3 Cl(g)d)Al3+(g) + 3 Cl-(g) --> AlCl3(s)e)Cl(g) + e- --> Cl-(g)
Answers
The correct representation for the lattice energy of AlCl3 is option (d): Al3+(g) + 3 Cl-(g) → AlCl3(s).
Lattice energy is the energy released when gaseous ions combine to form a solid ionic lattice. In the case of AlCl3, the reaction that represents the formation of the ionic lattice is the combination of Al3+ ions and Cl- ions to form solid AlCl3.
Option (a), Al(s) + 3/2 Cl2(g) → AlCl3(s), represents the formation of AlCl3 from its elements, but it does not represent the lattice energy specifically. It shows the formation of the compound, but not the process of ionic lattice formation.
Option (b), Al(s) → Al(g), represents the sublimation of aluminum, which is not directly related to the formation of AlCl3 or the lattice energy.
Option (c), 3/2 Cl2(g) → 3 Cl(g), represents the dissociation of chlorine molecules into chlorine atoms, which is not directly related to the formation of AlCl3 or the lattice energy.
Option (e), Cl(g) + e- → Cl-(g), represents the process of electron capture by chlorine, which is not directly related to the formation of AlCl3 or the lattice energy.
Therefore, option (d) correctly represents the formation of AlCl3 through the combination of gaseous Al3+ ions and Cl- ions, which corresponds to the lattice energy of AlCl3.
Know more about Lattice Energy here:
https://brainly.com/question/29735933
#SPJ11
NEED HELPP ASAP DUE SOONN CHEMISTRY

Answers
The final temperature of both systems is 32.7°C and the initial temperature of the metal was 100°C. Thus, its temperature change is 67.3 °C. Similarly the temperature change for water is then 10.2°C. The specific heat of the metal is 0.45 J/ °Cg.
What is calorimetry ?Calorimetry is an analytical technique used to determine the change in heat energy absorbed or evolved by a physical or chemical change of a substance.
The calorimetric equation connecting the heat energy q with the mass m, specific heat c and temperature difference ΔT is :
q = m c ΔT.
The initial temperature of metal was 100°C and the final temperature is 32.7 °C. Thus the temperature change is 100 - 32.7 °C = 67.3 °C.
Similarly temperature change of water which was having an initial temperature of 22.5 °C is 32.7 - 22.5 °C = 10 °C
To find the specific heat c of the metal, equate heat lost by the metal with the heat gained by water where, the specific heat of water is 4.18 J/ °Cg.
70.3 g × 67.3°C × C = 50 g ×10.2°C × 4.18 J/ °Cg
C= 0.45 J/ °Cg.
Therefore, the specific heat of the metal is 0.45 J/ °Cg.
To find more on calorimetry, refer here:
https://brainly.com/question/11477213
#SPJ1
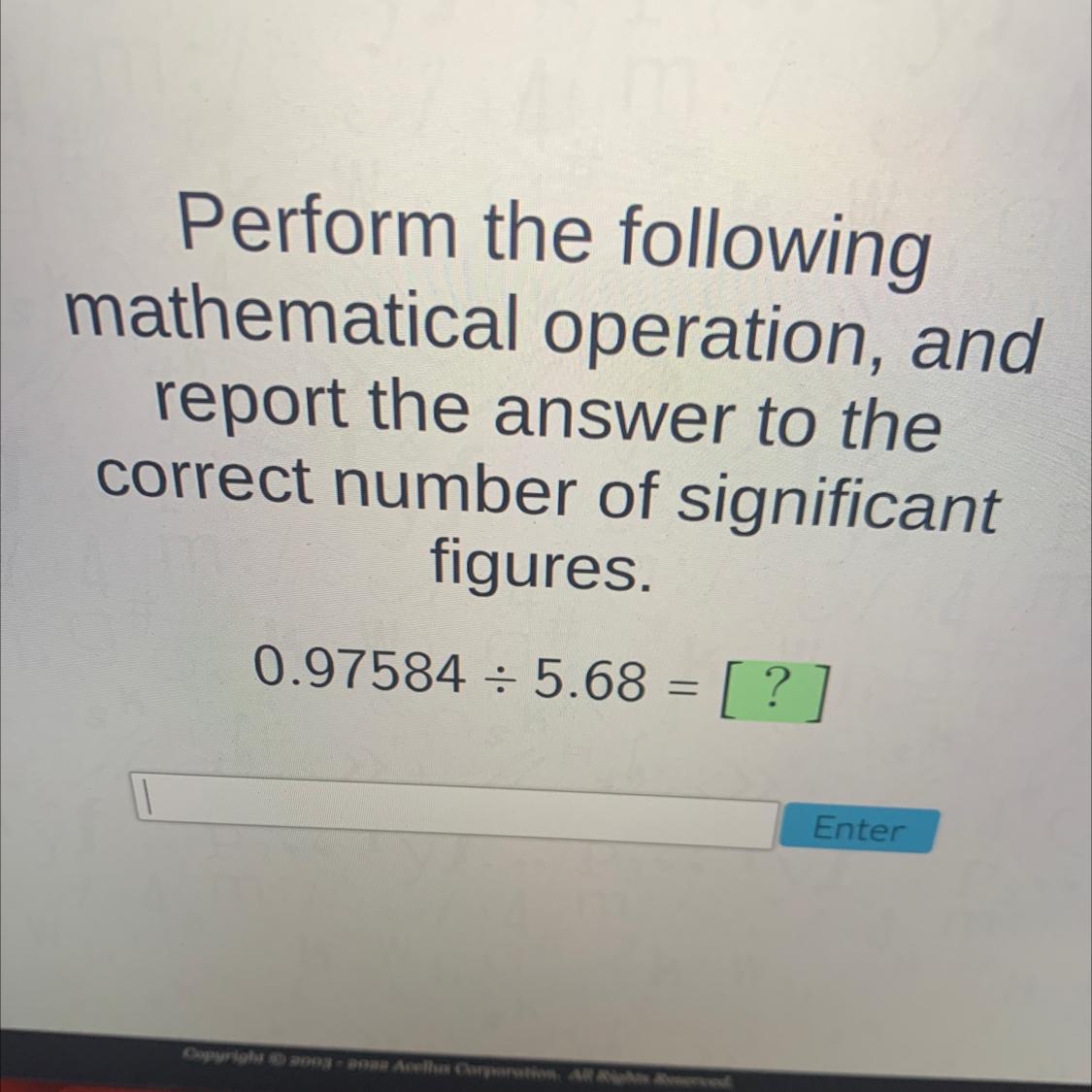
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.0.97584 = 5.68 = [ ?]=Enter
Answers
When we do the respective division operation we get the following result 0.171802.
When we do the respective division operation we get the following result 0.171802. But we must adjust the significant figures taking into account the number that has less significant figures, which is 5.68.
5.68 has three significant figures. So we will adjust the significant figures of the result to three.
0.97584 / 5.8 = 0.1718 = 0.172
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
C
Unit Test
Unit Test Review Active
G
If a person has the values for an object's density and volume, what value can be calculated?
the object's size
the object's mass
the shape the object forms in a container
the amount of space the object takes up
Answers
If a person has the values for an object's density and volume, they can calculate the object's mass. Hence option B) is correct.
If a person has the values for an object's density and volume, they can calculate the object's mass. Density is defined as the mass per unit volume of an object. Mathematically, density is calculated by dividing the mass of an object by its volume. Rearranging the equation, we find that mass is equal to the product of density and volume. Therefore, if the density and volume of an object are known, multiplying them together will yield the object's mass. The other options mentioned in the question are not directly calculated using density and volume. The object's size is a broader term that encompasses various dimensions and may not be specifically derived from density and volume alone. The shape the object forms in a container and the amount of space the object takes up are influenced by both the object's mass and its dimensions, which are not solely determined by density and volume. Therefore option B) is correct.
For more question on density
https://brainly.com/question/26364788
#SPJ11
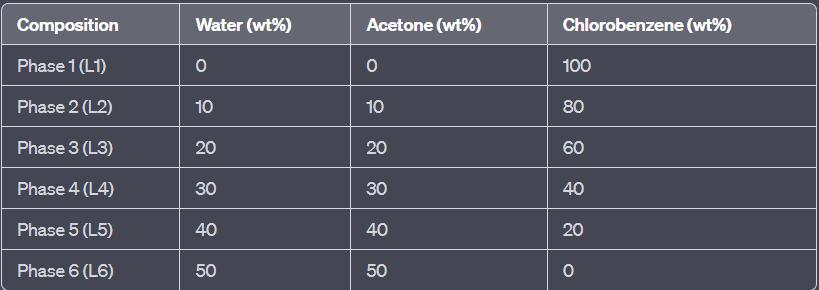
For the water + acetone + chlorobenzene system, construct the equilibrium diagram. Experimental data is shown in the table below. Plot the binodal curve, the critical point and the conjugation line eq
Answers
The equilibrium diagram for the water + acetone + chlorobenzene system includes the binodal curve, the critical point, and the conjugation line.
To construct the equilibrium diagram, we need experimental data, which is shown in the table attached below.
Now let's plot the equilibrium diagram:
Binodal curve:
The binodal curve represents the boundary between the liquid-liquid immiscibility region and the single-phase region. To plot the binodal curve, we connect the points corresponding to the compositions of the phases.
Critical point:
The critical point represents the highest temperature and pressure at which a liquid-liquid immiscible system can exist. To determine the critical point, we need additional experimental data, including temperature and pressure values for each composition.
Please provide the temperature and pressure values for the experimental data, or specify if they are not available.
Conjugation line:
The conjugation line represents the boundary between the liquid-liquid immiscibility region and the liquid-vapor immiscibility region. It is determined by finding the compositions where the phases exhibit the maximum difference in boiling points.
Once again, we need additional data, specifically the boiling points of the mixtures at each composition. Please provide the boiling point data or specify if it is not available.
To construct the equilibrium diagram for the water + acetone + chlorobenzene system, we require additional information such as temperature, pressure, and boiling point data.
Once we have this data, we can plot the binodal curve, critical point, and conjugation line, providing a comprehensive representation of the system's phase behavior.
For the water + acetone + chlorobenzene system, construct the equilibrium diagram. Experimental data is shown in the table below. Plot the binodal curve, the critical point and the conjugation line equilibrium concentration of the coexisting phases (mass fraction) aqueous phase organic phase water acetone chlorbenzene water acetone chlorbenzene 0.9989 (0) 0.0011 0.0018 0 0.9982 0.8979 0.1 0.0021 0.0049 0.1079 0.8872 0.7969 0.2 0.0031 0.0079 0.2223 0.7698 0.6942 0.3 0.0058 0.0172 0.3748 0.608 0.5864 0.4 0.0136 0.0305 0.4944 0.4751 0.4628 0.5 0.0372 0.0724 0.5919 0.3357 0.2741 0.6 0.1259 0.2285 0.6107 0.1608 0.2566 0.6058 0.1376 0.2566 0.6058 0.1376
To learn more about equilibrium, visit
https://brainly.com/question/30772553
#SPJ11
An empty beaker weighs 20g and when filled with
Kensene weighs 60g. If the Volume of the kerosene is 15cm2 calculate
the density of the kerosene
Answers
Answer: The density of kerosene is \(2.7g/cm^3\)
Explanation:
Density is defined as the mass contained per unit volume.
\(Density=\frac{mass}{Volume}\)
Given : Mass of kerosene = mass of beaker with kerosene - mass of empty beaker = 60 g - 20 g = 40 g
Volume of kersone = \(15cm^3\)
Putting in the values we get:
\(Density=\frac{40g}{15cm^3}\)
\(Density=2.7g/cm^3\)
Thus the density of kerosene is \(2.7g/cm^3\)
It is given that the probability of requiring ICU care for patients hospitalized for COVID-19 is 5%. What is the probability of observing at least one ICU admissions, among the next six patients hospitalized for COVID-19?| Answer: a. 0.26491 b. 0.73509 c. 0.03125 d. 0.98437 e. 0.23213
Answers
The probability of observing at least one ICU admission, among the next six patients hospitalized for COVID-19 is 0.26491. Option A is correct.
The probability of not observing any ICU admission among the next six patients hospitalized for COVID-19:
Probability of not observing ICU admission = 1 - Probability of observing ICU admission
= 1 - 0.05
= 0.95
Probability of no ICU admission among the next six patients hospitalized for COVID-19:
Probability of no ICU admission in one trial = 0.95
Probability of no ICU admission in six trials (patients)
= 0.95 × 0.95 × 0.95 × 0.95 × 0.95 × 0.95
= 0.73509
Now, we can calculate the probability of observing at least one ICU admission among the next six patients hospitalized for COVID-19:
Probability of at least one ICU admission= 1 - Probability of no ICU admission
= 1 - 0.73509
= 0.26491
Therefore, the answer is A.
Learn more about patients -
brainly.com/question/31288405
#SPJ11
Write the condensed formulas and provide IUPAC name for the following compound:
(a ethyl alcohol (in beverages)
Answers
The condensed formula and IUPAC nomenclature for the chemical ethyl alcohol are \(C_{2} H_{6} O\).
The condensed formula approach is what?Condensed structural formulae, which conserve space and are easier and faster to type out, display the atoms' positions similarly to a structural formula but on a single line. When demonstrating how a group of atoms in a molecule are linked to a single atom, condensed structural formulae are also helpful.
How do you tell whether a formula has been compressed?The hydrogens (or other ions or groups) that are connected to the carbon atoms are immediately next to one another in condensed chemical formulations. The corners and endpoints of lines are assumed to have carbon atoms in line-angle formulae. As each carbon atom is connected to four hydrogen molecules in total, it is assumed that each one has four bonds.
To know more about condensed formulas visit:
https://brainly.com/question/16700554
#SPJ1
Why do you think it is difficult to tell that a plate beneath it moving right now
Answers
Al + Fe3O4
Synthesis
Single replacement
Double replacement
O Decomposition
Combustion
-> Al₂O3 + Fe
Answers
Answer: Single Displacement
Explanation:
A bit confused about the set-up but if we're given Al + Fe3O4 -> Al₂O3 + Fe, Aluminum swaps places with Iron, giving us a Single Displacement reaction.
The true statements from the list are:
Hydrogen gas is a better reducing agent than tin metal.
Tin metal will reduce Cu2+ to copper metal.
The cell potential for a cell consisting of tin metal immersed in a tin(II) solution and the standard hydrogen electrode is not known because it was not measured in this experiment.
Copper metal will not reduce H+ to hydrogen gas.
Explanation:
Hydrogen gas is a better reducing agent than tin metal: This is because hydrogen gas has a lower reduction potential than tin metal. A reducing agent tends to lose electrons and get oxidized, and the lower the reduction potential, the more easily it can lose electrons and act as a reducing agent.
Zn2+ is the best oxidizing agent studied in this experiment: This statement is false. The best oxidizing agent is the one with the highest reduction potential, which is a measure of its ability to gain electrons and get reduced. The statement does not provide any comparison to other oxidizing agents, so it cannot be evaluated.
Tin metal will reduce Cu2+ to copper metal: This is a true statement. Tin metal has a higher reduction potential than Cu2+, so it can donate electrons to Cu2+ and reduce it to copper metal while getting oxidized to Sn2+.
Answers
The true statements from the list are 1. Hydrogen gas is a better reducing agent than tin metal, 2. Tin metal will reduce Cu2+ to copper metal and 3. Copper metal will not reduce H+ to hydrogen gas.
1. Hydrogen gas is a better-reducing agent than tin metal: This is true because hydrogen gas has a lower reduction potential than tin metal. A reducing agent tends to lose electrons and get oxidized, and the lower the reduction potential, the more easily it can lose electrons and act as a reducing agent.
2. Tin metal will reduce Cu2+ to copper metal: This is a true statement. Tin metal has a higher reduction potential than Cu2+, so it can donate electrons to Cu2+ and reduce it to copper metal while getting oxidized to Sn2+.
3. Copper metal will not reduce H+ to hydrogen gas: This is true because copper metal has a lower reduction potential than H+. Copper metal cannot donate electrons to H+ and reduce it to hydrogen gas since it is not strong enough reducing agent compared to hydrogen.
You can learn more about reducing agents at: brainly.com/question/2890416
#SPJ11
Ill give brainly thing
If your mass is 39 kg on Earth and you are on a moon that has 1/3 of the gravitational force of Earth what would your mass be on this moon?
Answers
Answer:
117 kg.
Explanation:
god luck
hydrogen peroxide is commonly used for multiple select question. disinfection of drinking water disinfection of medical equipment sterilization of diagnostic instruments disinfection of food preparation equipment skin and wound cleansing
Answers
Hydrogen peroxide is commonly used for the disinfection of drinking water, disinfection of medical equipment, sterilization of diagnostic instruments, and skin and wound cleansing.
Hydrogen peroxide (H₂O₂) is a versatile chemical compound with strong oxidizing properties, making it effective for various disinfection and sterilization purposes. It can be used in multiple settings for different applications.
Disinfection of drinking water: Hydrogen peroxide can be used as a disinfectant to kill bacteria, viruses, and other microorganisms in drinking water, ensuring its safety for consumption.
Disinfection of medical equipment: Hydrogen peroxide is commonly used in healthcare settings to disinfect medical equipment, such as surgical instruments and surfaces, to prevent the spread of infections.
Sterilization of diagnostic instruments: Hydrogen peroxide is also utilized for the sterilization of diagnostic instruments, such as endoscopes and ultrasound probes, to eliminate any potential pathogens and maintain a sterile environment for medical procedures.
Disinfection of food preparation equipment: In the food industry, hydrogen peroxide can be used to disinfect food preparation equipment, such as countertops, cutting boards, and utensils, to ensure food safety and prevent cross-contamination.
Skin and wound cleansing: Hydrogen peroxide is commonly used for cleaning and disinfecting skin and wounds. It can help remove debris, kill bacteria, and promote the healing process.
Overall, hydrogen peroxide's antimicrobial properties make it a valuable tool for various disinfection and sterilization purposes in different settings, contributing to maintaining hygiene and preventing the spread of infections.
To learn more about hydrogen peroxide, here
https://brainly.com/question/29102186
#SPJ4
Ginger wanted to see how long it would take to get to Brownsville from Houston. If it is 570 kilometers (km) to Brownsville, how long would it take her if she could only drive an average speed of 60 km/hour
Answers
Answer:
9.5 hours/ 9 hours 30 mins
Explanation:
Distance = speed × time so
Time = Distance ÷ speed
= 570 ÷ 60
= 9.5 hours
Why open flames near to ethanol can lead to serious fires?
Answers
\(\huge\bold\red{Aɴswᴇʀ}\)
Ethanol is a polar solvent, a flammable liquid made of gasoline and alcohol that fully and immediately combines with water instead of separating, which is the opposite of what a firefighter attempting to knock down a car fire might expect.
Answer:
Ethanol has a high vapor pressure. Therefore, even though you may not be able to see the particles of ethanol in the air, they are there and will readily fuel a combustion reaction if an open flame is nearby
Calculatethe yield of ATP whe 1 mole of stearic acid is completely oxidized to CO2 and H2O.Pretend that the person grading this problem is a smart high school student. Show all your work in a well-organized fashion so that the student gets it.
Answers
The yield of ATP when 1 mole of stearic acid is completely oxidized to CO₂ and H₂O is 147 moles of ATP.
To calculate the yield of ATP when 1 mole of stearic acid is completely oxidized, we need to consider the process of cellular respiration. Stearic acid is a fatty acid commonly found in animal fats and oils. During cellular respiration, fatty acids are broken down in a series of steps to produce energy in the form of ATP.
Stearic acid undergoes beta-oxidation, where it is broken down into two-carbon units called acetyl-CoA. Each acetyl-CoA molecule enters the citric acid cycle (also known as the Krebs cycle) and produces energy through a series of reactions.
In the citric acid cycle, one molecule of acetyl-CoA produces 3 molecules of NADH, 1 molecule of FADH₂, and 1 molecule of GTP (which can be converted to ATP). Each NADH molecule can produce 2.5 ATP molecules, and each FADH₂ molecule can produce 1.5 ATP molecules during oxidative phosphorylation.
Since stearic acid contains 18 carbon atoms, it can produce 9 molecules of acetyl-CoA. Therefore, the total yield of ATP from 1 mole of stearic acid is calculated as follows:
9 acetyl-CoA × (3 NADH × 2.5 ATP + 1 FADH₂ × 1.5 ATP + 1 GTP × 1 ATP) = 9 × (7.5 ATP + 1.5 ATP + 1 ATP) = 9 × 10 ATP = 90 ATP
However, this calculation does not take into account the energy required to activate fatty acids and transport NADH from the cytoplasm to the mitochondria. When these factors are considered, the actual yield of ATP from the complete oxidation of stearic acid is estimated to be around 147 moles of ATP.
Therefore, when 1 mole of stearic acid is completely oxidized to CO₂ and H₂O, it can yield approximately 147 moles of ATP.
learn more about cellular respiration here:
https://brainly.com/question/29760658
#SPJ11
The sample of oxygen gas is compressed from 5.25L to 1.75L. If the original pressure was 740. Torr, what is the final pressure?
Answers
Answer:
2220 Torr
Explanation:
Assuming constant temperature, we can solve this problem by using Boyle's law, which states that:
V₁P₁=V₂P₂Where subscript 1 refers to the initial volume and pressure, while subscript 2 refers to the final state, meaning that in this case:
V₁ = 5.25 LP₁ = 740 TorrV₂ = 1.75 LP₂ = ?We input the data:
5.25 L * 740 Torr = 1.75 L * P₂P₂ = 2220 TorrBurning wood in a fireplace heats the room around it. Is this an example of convection, conduction, or radiation
Answers
What is the chemical composition of PFAS?
Answers
Answer:
PFAS molecules are made up of a chain of linked carbon and fluorine atoms. Because the carbon-fluorine bond is one of the strongest, these chemicals do not degrade in the environment.
The National Stock Number (NSN) test search is used to locate test reports and Special Packaging Instructions (SP) on which of the following?
Answers
The NSN test search is used to locate test reports that provide comprehensive information about the testing and evaluation of specific products or items.
The National Stock Number (NSN) test search is used to locate test reports on various products or items. Test reports provide detailed information about the testing and evaluation of a particular product's performance, quality, safety, or compliance with specific standards or requirements. These reports are often conducted by independent testing laboratories or organizations to assess the characteristics and capabilities of the product.
The NSN test search allows users to search for test reports based on the NSN, which is a unique identification number assigned to each item in the federal supply system. By inputting the NSN into the search, individuals or organizations can access relevant test reports associated with that specific item or product.
The test reports obtained through the NSN test search can be valuable for various purposes, such as quality assurance, product development, procurement decisions, or regulatory compliance. They provide important insights into the performance and reliability of the item being tested, allowing users to make informed decisions based on objective evaluation and testing data.
In summary, the NSN test search is used to locate test reports that provide comprehensive information about the testing and evaluation of specific products or items.
Learn more about testing from below link
https://brainly.com/question/27794277
#SPJ11
Are the Nobel gases reactive?
Answers
Answer:
The noble gases are relatively nonreactive. In fact, they are the least reactive elements on the periodic table. This is because they have a complete valence shell. They have little tendency to gain or lose electrons.
Explanation:
Explanation:
As you may or may not know, atoms of elements take and give electrons in order to form bonds and, therefore, compounds. However, noble gases have a full valence shell (8 electrons in the valence shell).
Normally, in an atomic reaction, there is an instability in the atoms' valence shells. For example, oxygen has only 6 valence electrons. In order for an atom to be completely stable it needs to have 8 valence electrons. So, two hydrogen atoms with one electron each bond with the oxygen atom, creating a stabilization.
However, in the case of noble gases, their atoms already have a full valence shell, so there is no instability and no need to form bonds with other elements. In fact, noble gases got their name from their inactivity with other elements.
Based on the oxidation states of the atoms in this reaction, answer the questions. 4Fe(0) + 3O2(0) → 2Fe2(3+)O3(2-) How many electrons does the iron half-reaction lose? How many electrons does the oxygen half-reaction gain? What is the total number of electrons that are moved in this oxidation-reduction reaction?
Answers
Answer:
Twelve electrons
Explanation:
If we look at the product side, we will notice that iron lost twelve electrons and oxygen gained twelve electrons.
Hence, we have 4Fe^3+ and 2O3^2- showing twelve electrons lost/gained.
Answer:
How many electrons does the iron half-reaction lose?
12
How many electrons does the oxygen half-reaction gain?
12
What is the total number of electrons that are moved in this oxidation-reduction reaction? 12
Explanation:
Can anyone help me with all of these?

Answers
The frequency and the wavelength are;
1) 4 * 10^-5 m
2) 2 * 10^-7 m
3) 4.4 * 10^13 Hz
4) 4.6 * 10^14 Hz
5) 4.5 * 10^-7 m
What is the frequency of light?From the fact that;
c = λf
c = Speed of light
λ = wavelength
f = frequency
1) λ = 3 * 10^8/7.5 * 10^12
λ = 4 * 10^-5 m
2) λ = 3 * 10^8/1.5 * 10^15
λ = 2 * 10^-7 m
3) f = c/ λ
f = 3 * 10^8/6.8 * 10^-5
f = 4.4 * 10^13 Hz
4) f = 3 * 10^8/6.5 * 10^-7
f = 4.6 * 10^14 Hz
5) λ = c/f
= 3 * 10^8/6.6 * 10^14
= 4.5 * 10^-7 m
Learn more about wavelength:https://brainly.com/question/13533093
#SPJ1
what is soil? what is it composed of? explain how weathering (both physical and chemical) cause soil formation (see attached pdf for more information) 2. soil profiles: include horizons o, a, e, b, c, r and a description of each horizon 3. soil textures: compare and contrast sand, silt, and clay 4. soil permeability and porosity
Answers
Soil is a dynamic and diverse mixture of mineral particles, organic matter, water, air, and living organisms. Both physical and chemical weathering processes contribute to soil formation by breaking down rocks into smaller particles. Soil profiles consist of different horizons, each with distinct characteristics. Soil texture influences its fertility and water-holding capacity. Soil permeability and porosity affect water movement and availability to plants.
Soil is a complex natural resource that forms through the weathering of rocks and the accumulation of organic matter over time. It is composed of mineral particles, organic matter, water, air, and living organisms.
Weathering plays a crucial role in soil formation. Physical weathering involves the mechanical breakdown of rocks into smaller fragments through processes such as freeze-thaw cycles, abrasion, and root action. Chemical weathering, on the other hand, involves the alteration of minerals through chemical reactions, including dissolution, oxidation, and hydrolysis. These weathering processes break down rocks into smaller particles, contributing to the formation of soil.
Soil profiles are vertical sections of soil that display distinct layers called horizons. The commonly observed horizons include O, A, E, B, C, and R. The O horizon is the organic layer consisting of decomposed organic matter. The A horizon, or topsoil, is rich in organic material and is the most fertile layer. The E horizon is a zone of leaching, where minerals and nutrients are washed out. The B horizon is the subsoil layer, containing minerals leached from above. The C horizon consists of weathered parent material, while the R horizon represents the bedrock.
Soil textures refer to the proportions of sand, silt, and clay particles in a soil sample. Sand particles are the largest and have low water-holding capacity but provide good drainage. Silt particles are medium-sized and have moderate water-holding capacity. Clay particles are the smallest and have high water-holding capacity but poor drainage. Soil texture affects the soil's fertility, water retention, and drainage properties.
Soil permeability refers to how easily water can flow through the soil. It is influenced by the soil texture and structure. Sandy soils have high permeability, allowing water to flow through quickly, while clay soils have low permeability, causing water to move slowly. Porosity refers to the amount of pore space in the soil, which determines its ability to hold water and air. Sandy soils have high porosity due to large particle sizes, while clay soils have lower porosity due to small particle sizes and high compaction.
Learn more about sandy soil here:
https://brainly.com/question/30997548
#SPJ4
What holds more salt warm or cold water?
Answers
in a hydrogen atom which transition would emit the longest wavelength light? select all that apply 1. n
Answers
The wavelength will be longer the higher the magnitude of the fundamental quantum number to which the electron is hopping. Thus, the longest wavelength is produced by the n = 6 to p = 8 transition.
How would one describe wavelengths?The length of a wave is expressed by its wavelength. The wavelength is the distance between one wave's crest and the following wave's crest. The wavelength can also be determined by measuring from the "trough" (bottom) of one wave to the "trough" of the following wave.
Why is wavelength significant and what is it?The distance that a wave repeats is measured by its wavelength. The letter lambda () stands for these recurrences, also known as wavelengths. Energy travels through a medium as waves (like the atmosphere, the vacuum, or even the ocean).
To know more about wavelength visit-
https://brainly.com/question/12924624
#SPJ4
Polyelectrolytes are typically used to separate oil and water in industrial applications. The separation process is dependent on controlling the pH. Fifteen (15) pH readings of wastewater following these processes were recorded. Is it reasonable to model these data using a normal distribution? 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 10.0 10.5 7.6 11.4 11.4 10.0 Yes, it passes the "fat pencil" test. Therefore, a normal distribution is a reasonable model. No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O Yes, it passes the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O No, it does not pass the "fat pencil" test. Therefore, a normal distribution is a reasonable model.
Answers
No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. Option B is the correct answer.
The "fat pencil" test is a quick visual check to determine if a dataset can be reasonably approximated by a normal distribution. In this case, the pH readings of wastewater show a significant deviation from a normal distribution. The presence of several low pH values (1.0) and a few high pH values (10.0, 10.5, 11.4) indicate a non-normal distribution with skewness and potential outliers. Therefore, it is not reasonable to model these data using a normal distribution.
Option B is the correct answer.
You can learn more about normal distribution at
https://brainly.com/question/4079902
#SPJ11