Answers
The pH of the solution is 4.58.
What is pH?
pH which denotes "potential of hydrogen", is described as a scale used to specify the acidity or basicity of an aqueous solution.
In order to find the pH of the solution, we need to use the weak base-strong acid equation:
Kb = Kw / Ka = [CH3COO-][H+] / [CH3COOH]\
Ka = [H+][CH3COOH] / [CH3COO-]
[H+] = sqrt(Ka * [CH3COOH]) = √t(1.75x10^-5 * 0.15) = 1.36x10^-5 M
We then find the concentration of acetate ions from the sodium acetate.
We have 2.05 g of sodium acetate, which is equivalent to 2.05 / (82.03 + (12 + 16 + 1) * 2) = 0.0163 moles.
[CH3COO-] = 0.0163 M
Kb = Kw / Ka = 1.36x10^-5 / 0.0163 = 8.36x10^-10
In conclusion,
pH = pKb + log([CH3COO-] / ([CH3COOH] + [CH3COO-]) = 4.58
Learn more about pH at: https://brainly.com/question/172153
#SPJ1
Related Questions
What is the pH of a solution that is 1.9×10−9 M in NaOH ?
Answers

How do ionic and molecular compounds behave in water?

Answers
Answer:
When ionic compounds dissolve in water they go through a process called dissociation, splitting into the ions that make them up. However, when you place covalent compounds in water, they typically do not dissolve but form a layer on top of the water.
name two luminous objects other than the sun
Answers
Answer: flame in a lamp, tube light, electric bulb
Explanation:
Select all the correct answers.
Which of these are examples of mixing and separating?
removing seed casings from grains
a soda bottle bubbling when it is opened
a bright copper statue turning green from weathering
removing salt from seawater
water decomposing to oxygen and hydrogen Select all the correct answers.
Which of these are examples of mixing and separating?
removing seed casings from grains
a soda bottle bubbling when it is opened
a bright copper statue turning green from weathering
removing salt from seawater
water decomposing to oxygen and hydrogen
Answers
Answer: Removing salt from sea water
Answer:
removing seed casings from grains
a soda bottle bubbling when it is opened
removing salt from seawater
1. A sample of commercial concentrated hydrochloric acid is 11.8 M HCl and has a density of 1.190 g/mL. Calculate (a). the mass % of HCI (b). the molality of HCI (c). the mole fraction of HCI
Answers
(a) The mass percent of HCl in the solution is approximately 36.1%.
(b) The molality of HCl in the solution is approximately 15.5 mol/kg.
(c) The mole fraction of HCl in the solution is approximately 0.218.
(a) To calculate the mass percent of HCl, we need to determine the mass of HCl in a given volume of the solution.
Given: Concentration of HCl = 11.8 M
Density of the solution = 1.190 g/mL
First, we need to calculate the mass of the solution. Since density is mass per unit volume, the mass of 1 mL of the solution is 1.190 g.
Next, we need to calculate the mass of HCl in 1 mL of the solution. Since the concentration is given in moles per liter (M), and the molar mass of HCl is 36.46 g/mol, we can calculate the mass of HCl in 1 mL as follows:
Mass of HCl = concentration × volume × molar mass
= 11.8 mol/L × 0.001 L × 36.46 g/mol
= 0.430 g
Now, we can calculate the mass percent of HCl using the following formula:
Mass percent = (mass of solute ÷ mass of solution) × 100
= (0.430 g ÷ 1.190 g) × 100
≈ 36.1%
(b) The molality of HCl is calculated by dividing the moles of solute (HCl) by the mass of the solvent (water) in kilograms.
Since the density of the solution is given as 1.190 g/mL, the mass of 1 mL of the solution is 1.190 g. However, we need to consider the density of the solvent (water) to calculate the mass of water in the solution.
Assuming the density of water is 1 g/mL, the mass of water in 1 mL of the solution is (1.190 g - 0.430 g) = 0.760 g.
To calculate the molality of HCl, we need to convert the mass of water to kilograms:
Mass of water (kg) = 0.760 g ÷ 1000 = 0.000760 kg
The molality (m) is calculated using the formula:
Molality = (moles of solute ÷ mass of solvent in kg)
= (11.8 mol/L × 0.001 L) ÷ 0.000760 kg
≈ 15.5 mol/kg
(c) The mole fraction (X) of HCl is calculated by dividing the moles of HCl by the total moles of all components in the solution.
To calculate the mole fraction, we need to consider the volume of the solution and convert it to liters.
Given: Concentration of HCl = 11.8 M
Volume of the solution = 1 mL
Volume of the solution (L) = 1 mL ÷ 1000 = 0.001 L
To calculate the mole fraction of HCl, we need to calculate the moles of HCl and the moles of water (solvent) in the solution.
Moles of HCl = concentration × volume
= 11.8 mol/L × 0.001 L
= 0.0118 mol
Moles of water = mass of water ÷ molar mass of water
= 0.760 g ÷ 18.015 g/mol (molar mass of water)
= 0.0422 mol
Total moles in the solution = moles of HCl + moles of water
= 0.0118 mol + 0.0422 mol
= 0.054 mol
Mole fraction of HCl = moles of HCl ÷ total moles
= 0.0118 mol ÷ 0.054 mol
≈ 0.218
For such more questions on molality
https://brainly.com/question/14366957
#SPJ8
Ca(NO3)2(aq) + KCl(aq) →
Express your answer as a chemical equation.
Answers
The chemical equation is
\(\rm Ca(NO_ 3)_2 (aq) + 2KCl (aq) \rightarrow CaCl_2 + 2KNO_3\)
What are chemical equation?Chemical equation is defined as a symbolic representation of a chemical reaction in the form of symbols, in which the reactants and products are expressed in terms of their respective chemical formulas. It can also be called as chemical reactions.
There are basically six types of chemical reactions.
Combination reactionDecomposition reactionPrecipitation reactionNeutralization reactionCombustion reactionDisplacement reactionThus, the chemical equation is
\(\rm Ca(NO_ 3)_2 (aq) + 2KCl (aq) \rightarrow CaCl_2 + 2KNO_3\)
To learn more about chemical equations, refer to the link below:
https://brainly.com/question/28294176
#SPJ2
if the body is moving with uniform acceleration then, eng of motion are given as s = u+v/2+t
Answers
Yes, s = u+v/2+t, where s is the displacement, u is the beginning velocity, v is the end velocity, and t is the time required, is the equation of motion for a body travelling with uniform acceleration.
The basic law of motion, which states that a body's rate of change in displacement is directly proportional to its velocity, provides the basis for this equation. The equation of motion for a body travelling with constant acceleration, s = ut + 1/2at2, may be used to derive it.
The equation of motion for a body travelling with uniform acceleration is given by replacing the value of an as (v-u)/t and getting s = u+v/2+t. This formula is only accurate when the body's acceleration is constant and uniform.
Learn more about acceleration at:
https://brainly.com/question/12550364
#SPJ1
Which of the following chemical formulas show three molecules of a compound that has one
NEED FAST
atom of mercury and two atoms of bromine
1. 3Mc2Br
2. Hg3Br2
3. 3McBr2
4.McBr23
5.3HgBr2
6.3Hg2Br
Answers
The chemical formula : 3HgBr₂(Mercury(II) bromide)
Further explanationGiven
The chemical formulas of Mercury and Bromine
Required
The appropriate chemical formula
Solution
A molecular formula is a formula that shows the number of atomic elements that make up a compound.
The number of molecules is determined by the coefficient in front of the compound
the number of atoms is determined by the subscript after the atom and the coefficient
Three molecules⇒ coefficient = 3
one atom of Mercury ⇒Hg
two atoms of Bromine ⇒ Br₂
The chemical formula : 3HgBr₂
Shown above is the phase diagram for water as it is heated. Which section represents the phase of water with the highest kinetic energy?
Answers
The section that represents the phase of water with the highest kinetic energy is the gas phase or vapor phase.
Gas phase or vapor phase section is above the boiling point curve, which separates the liquid and gas phases. At this point, the temperature is at or above 100°C (at standard atmospheric pressure), and the kinetic energy of the water molecules is sufficient to overcome the intermolecular forces holding them in the liquid phase and escape into the gas phase. The gas phase has the highest kinetic energy because the water molecules in this phase are more widely separated and move more rapidly than in the liquid or solid phases. The gas phase is also characterized by the highest entropy or disorder, as the molecules are free to move in any direction and occupy a large volume. The section that represents the phase of water with the highest kinetic energy is gas phase or vapor phase.
for more questions on water
https://brainly.com/question/19491767
#SPJ11
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
7. What is the volume of the
composite
solid?
4 in.
3 in.
3 in.

Answers
Answer:
The volume of Component 1 is 36 cubic inches.
Explanation:
To calculate the volume of a composite solid, we need to determine the individual volumes of the different components and then add them together.
In this case, the composite solid consists of multiple components with the following dimensions:
Component 1:
Length: 4 inches
Width: 3 inches
Height: 3 inches
To find the volume of Component 1, we multiply the length, width, and height together:
Volume of Component 1 = Length x Width x Height = 4 in x 3 in x 3 in = 36 cubic inches
Therefore, the volume of Component 1 is 36 cubic inches.
Please provide the dimensions of the remaining components of the composite solid, and I will calculate the total volume by summing up the individual volumes.
please help fast :/
how many moles are present in 60 grams of hydrochloric acid , HCI ?
a : 1.40 moles
b : 36.45 moles
c : 1.65 moles
d : 60 moles
Answers
Answer: C- 1.65 moles
Explanation:
HCl = 1 + 35.45 = 36.45 g/mol
Glucose, C6H12O6, is used to prepare intravenous feeding solutions. What volume of 5.0 % W/V glucose solution can be prepared using 125 g of glucose? Show your working.
Please if the answer is correct, ill give brainliest
Answers
250 L of 5.0% w/v glucose solution can be prepared using 125 g of glucose.
We use the below formula to solve our problem,w/v = [ mass of solute (g) / volume of solution (mL) ] × 100
Substitute the values from our problem,5.0 % w/v = [ 125 g / volume of solution (mL) ] × 100
Rearranging the formula, we havevolume of solution (mL) = [ 125 g / 5.0 % w/v ] x 100
Substitute further for w/v,volume of solution (mL) = [ 125 g / (5.0 / 100) ] x 100
Simplify the expression,volume of solution (mL) = [ 125 g / 0.05 ] x 100
Hence, the volume of solution (mL) = 250,000 mL or 250 LCalcium carbonate and copper sulfate are each mixed into a beaker of water.
After a few minutes the calcium carbonate settles on the bottom of its beaker,
while the copper sulfate stays dissolved. Which statement is true about this
experiment?
A. Copper sulfate is a solvent.
B. Calcium carbonate in water is a solution.
C. Copper sulfate in water is a solution.
D. Calcium carbonate is a solvent.
Answers
If 10 moles of a gas are at a pressure of 3.6 atm and at a temperature of 27°C, what is the volume of the container that the gas is in.
Answers
Answer:
68.4 liters
Explanation:
PV = nR T R = gas constant = .082057 L atm / K-mole
n = 10 T = 27C = 300.15 K
3.6 V = 10 * .082057 * 300.15 solve for V = 68.4 liters
How can or organism's genetic traits affect its ability to survive?
Answers
Answer:
According to Charles Darwin's theory of evolution by natural selection, organisms that possess heritable traits that enable them to better adapt to their environment compared with other members of their species will be more likely to survive, reproduce, and pass more of their genes on to the next generation.
How is this correct.how is this not wrong.Did you get it right.If you did explained please.
Answers
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
What is the mass of 2.7 x 10^18 molecules of HBr? (molar mass of HBr = 80.912 g/mol)
Answers
Answer:3.6 X 10-4 g
Explanation:
2.7 X 10^18 X (1 mole / (6.02 X 10^23) X ( 80.912 g / 1 mole) = 3.629 X 10-4 g HBr. If needed to correct Sig Figs 3.6 X 10-4 g HBr
What is the best way to measure the pH of a natural solution while out in a forest?
Answers
The best way to measure the pH of a natural solution while out in a forest is to use a portable pH meter or pH test strips specifically designed for field use. These instruments provide accurate and reliable pH measurements and are convenient for outdoor applications.
1. Prepare the necessary equipment: Before heading out to the forest, gather the required tools. You will need a portable pH meter or pH test strips, as well as the necessary reagents or calibration solutions if using a pH meter.
2. Collect the sample: Locate the natural solution you want to measure the pH of, such as a stream, pond, or soil. Use a clean container to collect a representative sample of the solution.
3. Calibrate the pH meter (if applicable): If you are using a portable pH meter, it is essential to calibrate it before taking measurements. Follow the manufacturer's instructions to calibrate the meter using the provided calibration solutions.
4. Conduct the measurement: For pH meters, immerse the electrode into the collected sample. Allow some time for the reading to stabilize, and then record the pH value indicated on the meter's display.
5. Using pH test strips: If you are using pH test strips, dip the strip into the collected sample for the recommended amount of time. Remove the strip and compare the color change with the provided color chart. Determine the corresponding pH value from the chart.
6. Repeat for accuracy: To ensure reliability, repeat the measurement process at least once and compare the results. This step helps confirm the accuracy of your measurements.
7. Record and analyze the data: Note down the pH values obtained and any relevant observations. Analyze the data as needed for your research or monitoring purposes.
By following these steps and using the appropriate equipment, you can effectively measure the pH of a natural solution while in a forest setting.
For more such questions on measurements, click on:
https://brainly.com/question/28391278
#SPJ8
What describes how gas particles collide?
*
1 point
Collisions between gas particles and the walls of a container.
Gas particles do not attract or repel one another.
Gas particles are in constant random motion.
Collisions are perfectly elastic.
The space between gas particles is larger than in other phases.
Average kinetic energy of the gas particles.
Answers
Gas particles are in constant random motion and collisions between them are perfectly elastic.
This means that the kinetic energy of the particles is conserved during collisions with one another and that they can bounce off of each other without losing energy.Collisions between gas particles are perfectly elastic, meaning that no energy is lost in the collision and the kinetic energy of the particles is conserved. The space between gas particles is larger than in other phases, which allows them to move around freely and not be strongly affected by the other particles. The average kinetic energy of the gas particles is determined by the temperature of the system.
learn more about collisions Refer:brainly.com/question/11647605
#SPJ1
I'll give you 100 points
Which are evidence of seafloor spreading? Select three options. A.molten material B.magnetic stripes C.continent material D.drilled core samples E.ocean water samples
Answers
B. Magnetic stripes
D. Drilled core samples
What is a system called when neither energy nor matter is exchanged between the system and the surroundings?
Closed system
Free energy
Isolated system
Open system
Answers
Answer:
open system
Explanation:
Answer:
Isolated system
Explanation:
An isolated system is one that cannot exchange either matter or energy with its surroundings.
substances that release protons when they dissolve in water are termed bases and result in a ph higher than 7.
Answers
Bases are substances that, when dissolved in water, release protons and drop the pH below 7. Acids are substances that produce protons when they dissolve in water, lowering the pH below 7.
When an object dissolves in water, it releases a proton (H +), which then reacts with the water molecule (H2O) to form the hydroxonium ion (H3O +). Acids also go below the pH 7 threshold. H+ and OH- concentrations are balanced at pH 7. Anything with a pH of 7 or lower is acidic, whereas anything with a pH of 7 or higher is basic. A pH change of one unit corresponds to a ten-fold rise or fall in the concentration of hydrogen ions.
To learn more about base click here https://brainly.com/question/27089588
#SPJ4
complete question: Regarding acids, bases, and pH, which of these statements is true? Choose one: A. Substances that release protons when they dissolve in water are termed acids and result in a pH lower than 7. B. Substances that release protons when they dissolve in water are termed bases and result in a pH lower than 7. C. Substances that release protons when they dissolve in water are termed bases and result in a pH higher than 7. D. Substances that release protons when they dissolve in water are termed acids and result in a pH higher than 7.
An ideal gas mixture contains 5 moles N2 and 15 moles O2. The total pressure in the container is 40 atm. What is the partial pressure of N2?
Answers
The partial pressure of the nitrogen gas is 10 atm.
What is the partial pressure?According to the Dalton's law, when we have a mixture of gases, the partial pressure of the gas is the pressure of one of the gases that we find in the system. Thus the total pressure of the system is the sum of all the partial pressures of all the gases.
We have the following information;
Total pressure of the gas = 40 atm
Total number of moles = moles of nitrogen + moles of oxygen = 15 mols + 5 mols
= 20 moles
Mole fraction of nitrogen gas = 5 moles
We now have;
Mole fraction * Total number of moles
= 5 moles/20 moles * 40 atm
= 10 atm
Learn more about partial pressure:https://brainly.com/question/13199169
#SPJ1
1. Making coffee or tea requires hot water to remove soluble components from the grounds or leaves. This is a separation process called extraction, removing a soluble component from solid material. What other separation technique is used to prepare the coffee or tea to drink
Answers
Answer:
The other technique which used to separate the coffee or tea is Filtration.
Filtration is a physical, biological or chemical operation that separates solid matter and fluid from a mixture with a filter medium that has a complex structure through which only the fluid can pass.
Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate.
3.
Carlos is trying to figure out the best place to put his router in order to get the best
signal. Which is not an independent variable?
A. Distance from the router
B. Signal strength at the computer
OUTPU
C. The number of walls between the router and computer
STATIO
D. The number of devices on the router
Answers
Answer:
right answer is option no c
Chose the element pairings that are likely to react with each other
Na and K or Na and Br
Answers
Answer:
Na k
Explanation:
because na is a metal and potassium is also a metal and both are active metal so is less likely to react as no bond is formed between them
Complete the table. Then explain how wavelength and frequency are mathematically related.
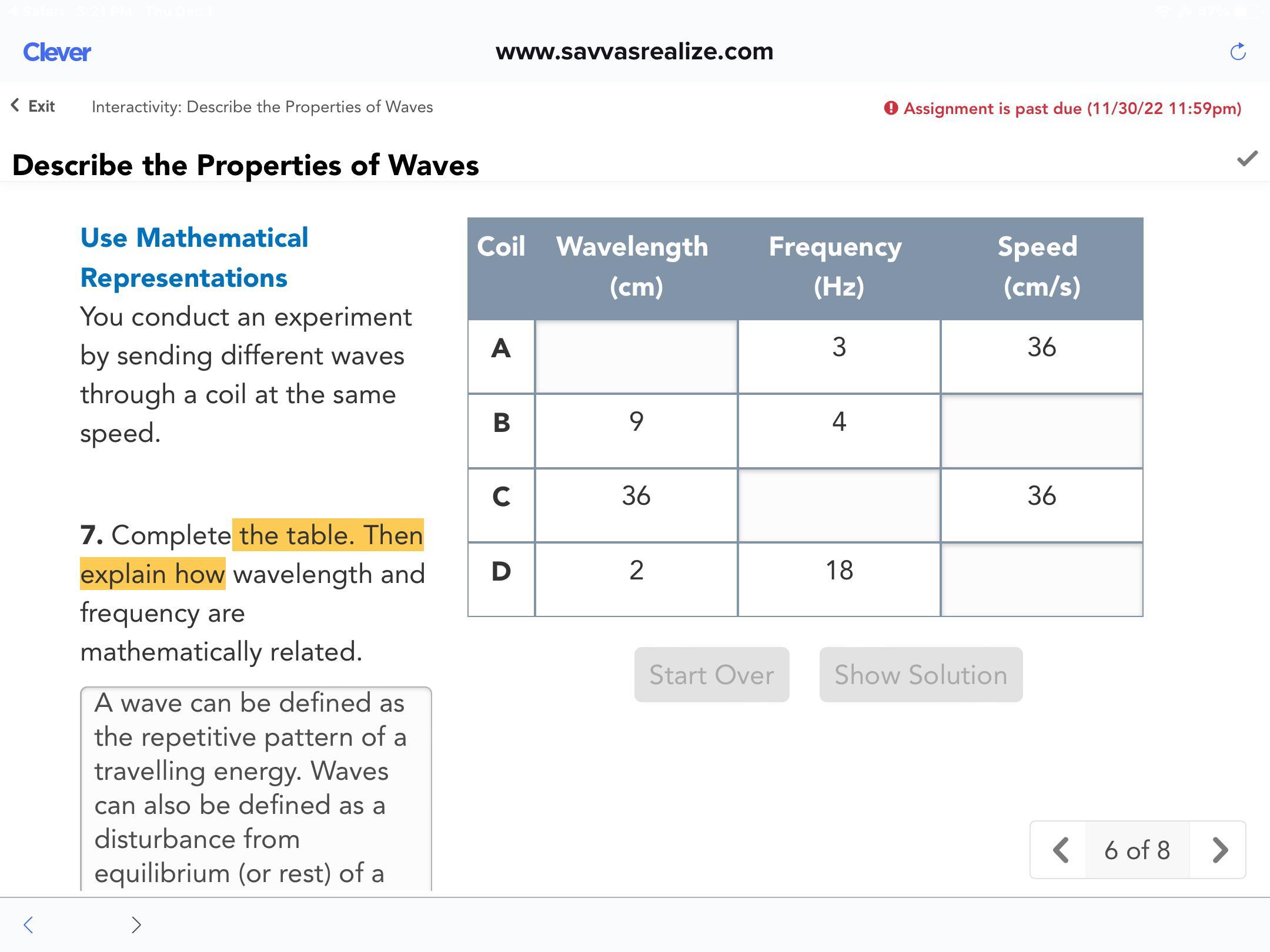
Answers
The wavelength is same.
What is wavelength?
Wavelength can be defined as the distance between two successive by throughs of a wave.
The question wants you to determine the energy that is the incoming photon by the must have in order to the accept the electron that melted it tob the jump to 2 to 1.
A good starting point here will be to calculate the energy of to the photon emitted when the electron falls from 1 to 2
by applying the Rydberg equation.
1/π = R
λ
si the wavelength of the emittted photon R
is the Rydberg constant, equal to 1.907.
This means that you haveλ=
4.10
So, you know that when an electron falls from the
to
a photon of wavelength by the
410 nm
is emitted. This implies by that in order to the for the electron by jump to the 2 to 1 .
it must absorb a photon of the same wavelength.
To know more about wavelentgh click-
https://brainly.com/question/10750459
#SPJ1
What state
of matter
exists in
area B?
A. gas
B. liquid
C. solid
Pressure
(atm)
61
6543210
0
50 100 150 200
Temperature (°C)
Answers
Considering the phase diagram, the state of matter that exists in area B is gas.
The correct option is A.
What is a phase diagram?A phase diagram is a graphical representation that shows the conditions of temperature and pressure at which different phases or states of a substance exist.
The axes of a phase diagram typically represent temperature (usually on the horizontal axis) and pressure (usually on the vertical axis). The diagram is divided into regions that correspond to different phases, and the lines separating these regions represent phase boundaries.
The point where three phase boundaries meet is known as the triple point, which represents the temperature and pressure at which all three phases can coexist in equilibrium.
Learn more about phase diagrams at: https://brainly.com/question/28097253
#SPJ1
