Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). If you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 330 mmHg, what is the pressure (in mmHg) of water vapor after the reaction has completed (temperature and volume do not change).
Answers
The pressure (in mmHg) of water vapour after the reaction has completed (temperature and volume do not change) is 1650 mmHg .
The balanced chemical equation for the given reaction is : 2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
From the balanced equation, we can see that for every 2 moles of butane combusted, 10 moles of water vapor are produced.
Given that the mixture of butane and oxygen is in the correct stoichiometric ratio, we can assume that all of the butane will react completely, and thus we have 2 moles of butane reacting.
Now, let's calculate the partial pressure of water vapor using the ideal gas law : PV = nRT
Where:
P is the pressure (in mmHg)
V is the volume (which we assume to be constant)
n is the number of moles
R is the ideal gas constant (0.0821 L·atm/(mol·K))
Since the volume and temperature are constant, we can write:
P1V = n1RT and P2V = n2RT
As we know that 2 moles of butane react to produce 10 moles of water vapor, we have:
n1 (butane) = 2 moles ; n2 (water vapor) = 10 moles
Since V and R are constant, we can divide both these equations to get:
P1/P2 = n1/n2
Substituting the known values:
P1/P2 = 2/10
= 1/5
Given that the total pressure of the mixture is 330 mmHg, the partial pressure of water vapor (P2) can be calculated as:
P2 = P1 / (1/5) = P1 * 5
Therefore, the pressure of water vapor after the reaction has completed is 5 times the pressure of the butane, which is:
P2 = 330 mmHg * 5 = 1650 mmHg
Thus, The pressure (in mmHg) of water vapour after the reaction has completed (temperature and volume do not change) is 1650 mmHg .
To learn more about pressure :
https://brainly.com/question/24719118
#SPJ11
Related Questions
A buffer solution is formed by mixing equal volumes of 0.12 M NH3(aq) and 0.10 M HCl(aq), which reduces the concentration of both solutions by one half. Based on the pKa data given in the table, which of the following gives the pH of the buffer solution?
pH=−log(0.050)=1.30
pH = 9.25 + log (
0.010
0.050
)
=
8.55
( 0.050
0.010
)=8.55
pH = 9.25 + log (
0.060
0.050
)
=
9.32
( 0.050
0.060
)=9.32
pH = 14.00 -(-log(0.010)) = 12.00
Answers
The pH of the buffer solution formed by the weak base NH3 and its conjugate acid NH4+ is 9.32.
Therefore, the correct answer is option (3).
What is the pH of the buffer solution?The buffer solution is formed by the weak base NH3 and its conjugate acid NH4+.
The equation for the reaction is: NH3(aq) + HCl(aq) -> NH4Cl(aq)
The concentrations of NH3 and NH4+ are the same, and the concentration of HCl is smaller. Therefore, the pH of the buffer will be closer to the pKa of the weak acid NH4+. The pKa of NH4+ is 9.24.
To calculate the pH of the buffer solution, we can use the Henderson-Hasselbalch equation:
pH = pKa + log([NH3]/[NH4+])
At equilibrium, the concentration of NH3 and NH4+ will be reduced by half, so:
[NH3] = 0.06 M / 2 = 0.03 M
[NH4+] = 0.05 M / 2 = 0.025 M
Substituting these values in the equation:
pH = 9.24 + log(0.03/0.025
pH = 9.32
Learn more about the pH of a buffer at: https://brainly.com/question/22390063
#SPJ1
Determine how many different monobromination products you expect from bromination of the following compound:
Answers
Only one monobromination products is expected from bromination of the compound:
What is Bromination ?When a substance undergoes bromination, bromine is added to the compound as a result of the chemical reaction. After bromination, the result will have different properties from the initial reactant.
An illustration of an electrophilic aromatic substitution reaction is the bromination of benzene. In this reaction, an intermediate is produced when the electrophile (bromine) makes a sigma bond with the benzene ring. The intermediate is then stripped of a proton to create a substituted benzene ring.Learn more about Bromination here:
https://brainly.com/question/24202507
#SPJ4
a set of dilutions ranging in concentration from 1x10-2 m to 2x10-2 m are used to prepare a standard curve. transmittances of the dilutions range from 12% to 88%. could this plot be used to determine the concentration of a sample that had a concentration of 1.0x10-3 m?
Answers
The correct option is (B) No, the solution is too dilute and would have a %T over 90% where Beer's Law does not apply.
The solution is too dilute, it will have lower than desired concentration of the desired substance. To make solution more concentrated, more of the desired substance must be added to the solution. This increases the concentration of the desired substance, allowing it to be more effective in the intended application.
The amount of the desired substance must be adjusted according to the desired concentration of the solution. In addition, the amount of other substances in the solution must be considered to ensure the desired concentration is not too high or too low. Too high concentration could produce an undesired reaction, while too low a concentration would decrease the efficiency of the desired reaction.
Full question:
A set of dilutions ranging in concentration from 1x10-2 M to 2x10-2 M are used to prepare a standard curve. Transmittances of the dilutions range from 12% to 88%. Could this plot be used to determine the concentration of a sample that had a concentration of 1.0x10-3 M?
A) No, the solution is too dilute and would have a %T under 10% where Beer's Law does not apply.
B) No, the solution is too dilute and would have a %T over 90% where Beer's Law does not apply.
C) Yes, simply extend the standard curve.
D) No, the solution is too concentrated and would have a %T over 90% where Beer's Law does not apply.
To know more about Dilute solution:
brainly.com/question/16926361
#SPJ4
An inflated balloon has a volume of 0.55 L at standard pressure of 1 atm and is allowed to rise a height of 6.5 km, where the pressure is about 0.9 atm. Assuming that the temperature remains constant, what is the final volume of the balloon?
Answers
The final volume of the balloon is 0.61 L
The Ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. The ideal gas equation can be written as-
PV = nRT
where,
P = Pressure
V = Volume
T = Temperature
n = number of moles
Given,
Initial volume = 0.55 L
Initial Pressure = 1 atm
Final pressure = 0.9 atm
P₁V₁ = P₂V₂
1 × 0.55 = 0.9 × V₂
V₂ = 0.61 L
Learn more about Ideal gas law, here:
https://brainly.com/question/12624936
#SPJ1
a 2.5 m solution of the acid ha has a ph of 1.20. what is the ka of the acid? the equation described by the ka value isha(aq) h2o(l)⇌a−(aq) h3o (aq)
Answers
The Ka of the acid is approximately 1.78 × 10^-5.
To solve this problem, we can use the relationship between the pH and the acid dissociation constant (Ka) of the acid:
pH = -log[H3O+]
Ka = [A-][H3O+] / [HA]
where [HA], [A-], and [H3O+] are the concentrations of the undissociated acid, the conjugate base, and the hydronium ion, respectively.
We are given a 2.5 M solution of the acid, which means that the initial concentration of HA is also 2.5 M. We can use the pH to calculate the concentration of H3O+:
pH = 1.20 = -log[H3O+]
[H3O+] = 10^-1.20 = 6.31 × 10^-2 M
At equilibrium, some of the HA will dissociate into A- and H3O+, but we don't know the extent of this dissociation or the equilibrium concentrations of the species. However, we can assume that the dissociation is small compared to the initial concentration of HA, which is a common assumption for weak acids.
If we let x be the concentration of A- and H3O+ at equilibrium, then we can write the equilibrium concentrations of the species in terms of x:
[HA] = 2.5 M - x
[A-] = x
[H3O+] = x
Substituting these expressions into the expression for Ka, we get:
Ka = [A-][H3O+] / [HA]
Ka = (x)(x) / (2.5 M - x)
Since we assume that x is small compared to 2.5 M, we can make the approximation 2.5 M - x ≈ 2.5 M. This simplifies the expression for Ka:
Ka = x^2 / 2.5 M
Now we can solve for x in terms of Ka:
x = sqrt(Ka × 2.5 M)
Substituting this expression for x back into the equation for Ka, we get:
Ka = x^2 / 2.5 M
Ka = (Ka × 2.5 M) / 2.5 M
Ka = sqrt(Ka × 2.5 M)^2 / 2.5 M
Ka = (Ka × 2.5 M) / (6.31 × 10^-2 M)
Solving for Ka, we get:
Ka = (6.31 × 10^-2 M) × (10^-1.20) / 2.5 M
Ka = 1.78 × 10^-5
Therefore, the Ka of the acid is approximately 1.78 × 10^-5.
Learn more about acid here:
https://brainly.com/question/14072179
#SPJ11
and Amides are derivatives of __________and_________O amines, esters O amines, carboxylic acidsO alkanes, aminesO carboxylic acids, alcohols O alcohols, carboxylic acids
Answers
Option (b) is correct. Amides are the derivative of amines and carboxylic acids.
Amides are the derivatives of carboxylic acid and amines. A carboxylic acid contains the −COOH functional group, and in an amide the −OH part of that group is replaced by an −NH2 group. So, amides contain the −CONH2 group. Any member of either of two classes of nitrogen-containing compounds related to ammonia and amines. The covalent amides are neutral or very weakly acidic substances formed by replacement of the hydroxyl group (OH) of an acid by an amino group NR2, in which R may represent a hydrogen atom or an organic combining group such as methyl, CH3. Among the amides of commercial importance are acetamide and dimethylformamide HCON(CH3)2, which are used as solvents, the sulfa drugs, and the nylons.
To learn more about Amides please visit:
https://brainly.com/question/11622057
#SPJ4
A student was carrying out an investigation into the rate of reaction of calcium carbonate with acid. They forgot to zero the balance each time they measured the mass of calcium carbonate. Give the type of error that this caused.
Answers
The error caused by forgetting to zero the balance each time the mass of calcium carbonate is measured is known as the cumulative systematic error or the additive systematic error.
When the balance is not zeroed before each measurement, it introduces a constant offset or bias to the measurements. This means that the measured masses will consistently be either higher or lower than the true values. Over multiple measurements, this error accumulates and becomes systematic, resulting in a consistent overestimation or underestimation of the mass of calcium carbonate.
The systematic error does not affect the precision of the measurements as the repeated measurements will likely yield similar values due to the consistent bias. However, it does introduce a significant deviation from the true values, impacting the accuracy of the experiment.
To minimize this error, it is crucial to zero the balance before each measurement, ensuring that the balance is calibrated and any constant offsets are eliminated. This allows for accurate and reliable measurements in the investigation of the rate of reaction of calcium carbonate with acid.
For more such questions on cumulative systematic error.
https://brainly.com/question/29994673
#SPJ8
2. In the reaction NO+NO₂ N₂O3, an experiment finds equilibrium concentrations of [NO] = 3.8 M, [NO₂] = 3.9 M, and [N203] = 1.3 M. What is the equilibrium
constant K K for this reaction?
Answers
The equilibrium concentration, Kc for the reaction is: 0.088.
What is equilibrium concentration?A chemical reaction is considered to be in a state of chemical equilibrium when both the reactants and the products are in a concentration that does not change over time anymore. The forward response rate and the backward reaction rate are equal in this condition.
The presence of the chemicals in equal proportions is not a requirement for equilibrium. The fact that both the forward and backward reactions proceed at the same pace indicates that the reaction has progressed to a stage where the concentrations of the reactant and product remain constant throughout time.
Kc= concentration of product divided by concentration of reactant
NO + NO₂ → N₂O₃
or, Kc = N₂O₃ / (No) (NO₂)
or, Kc= ( 1.3 )/{ (3.9) ×(3.8) }
or, Kc=0.088
To know more about equilibrium concentration refer to:
https://brainly.com/question/13414142
#SPJ1
Vaporization that takes place inside a liquid is called________.
Answers
Answer:
evaporation
Explanation:
Answer:
Boiling
Explanation:
When a substance transitions from liquid to gas, it's called evaporation if it's on the surface, and boiling if it's all throughout the liquid.
Picture boiling water in a pot: it starts on the bottom, and the bubbles go toward the top. Evaporation starts on the surface.
The carbon atom forms a part of all the major molecules found in living things. Which of the following doesnot contain carbon?
Answers
Hydrochloric acid or Hcl
What is the name of MnO? Explain how you determined the bond type and the steps you used to determine the naming convention for the compound.
Answers
Answer:
Magnesiumoxide
Explanation:
I don't know
Why do most experts say we should not stop using plastic altogether?
Answers
What are examples and non-examples of stars?
Answers
Answer:
Explanation:
Good Example of a Star- The sun, Star Clusters, The North Star
Bad Examples of a Star-Earth, Galaxies, Solar Systems
Examples of stars: Sun, Stars.
Non-examples of stars: Earth, Moon, Planets.
Why Sun is a star?The Sun, a 4.5 billion-year-old yellow dwarf star and the center of our solar system, is a hot, burning ball of hydrogen and helium. It is the only star in our solar system and is located approximately 93 million miles (150 million kilometers) from Earth. On our home planet, life as we know it would not be possible without the energy of the Sun.Despite being the hub of our solar system and crucial to our existence, the Sun is simply an average-sized star. There have been found stars up to 100 times bigger. Additionally, many solar systems contain multiple stars. Scientists can learn more about the workings of far-off stars by examining our Sun.As a star, the Sun doesn’t have any moons, but the planets and their moons orbit the Sun.To learn more about the sun, refer to https://brainly.com/question/15837114
#SPJ2
First Name * hey can some one help
lexi
Last Name *
tomson
Hour
1
2
3
5
7
8
What is density? *
1 point
How much space something takes up
How many particles are in a given space
How much something weighs
What color something is
If something is more dense, what does that mean? *
1 point
The particles are closer together
The particles are further apart
It weighs more
It weighs less
As the air molecules in the balloon and bottle warmed up what happened? *
1 point
They got closer together
They spread further apart
They didn't change at all
The same principles of molecule movement that occurred in the cups also occurred in the balloon and bottle. (Hint: Did the molecules move the same ways?) *
1 point
True
False
What happened to the bubble seal when the bottle was placed in warm/hot water? *
1 point
It popped
It shrank (got smaller)
It expanded (got bigger)

Answers
Answer:
1.VOLUME– The amount of space an object takes up. Basic Unit (SI Unit) for Solids – Cubic Meter, m3. LENGTH/DISTANCE – The measurement between two points.
2.one particle per cubic meter.
3.it means it is heavier
4.In a liquid the particles are still close together but a little further apart than in a solid.
5.In gases the particles are much further apart than in solids or liquids.
Explanation:
thats all i can type lol
which technology was essential for the development of the cell theory?
Answers
Answer: Microscope
Explanation: This is a kid answering :)
PLEASE HELP ME WITH THIS CHEMISTRY HOMEWORK!!! WILL GIVE BRAINLIEST!!! :)

Answers
Explanation:
Plugging into the following equations will give you the answer (the answer is the attached image):
\(pH+pOH=14\)
\(pH=-log_{10}([H^+])\)
\(pOH=-log_{10}([OH^-])\)
\([H^+][OH^-]=10^{-14}\)
\([H^+]=10^{-pH}\)
\([OH^-]=10^{-pOH}\)

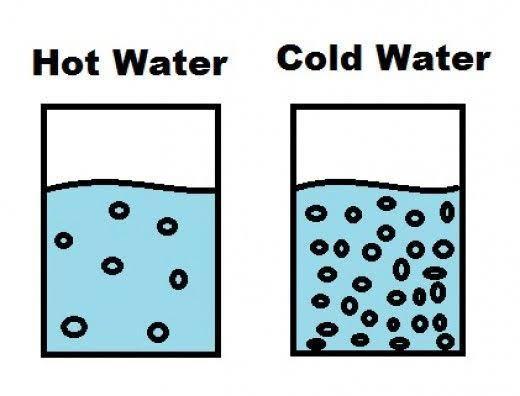
3- You saw a jar of hot water placed upside down over a jar of cold water. The hot
water stayed on top of the cold water without mixing. Why did the hot water stay on
top of the cold water?
Answers
Hot water stays on the top of cold water and doesn't mix. This is because of difference in DENSITY of water at different temperatures.
When we heat water, then the atoms gain energy from the heat and start moving away from each other. Now, when the atoms move away from each other than the (mass per unit volume) i.e. the density decreases. This makes water lighter.
When the temperature is less i.e. cold water then the atoms are more compactly packed and therefore the density of water is more at lower temperatures.
Since, hot water is lighter than cold water that is why it stays on top of the cold water.
How many atoms are in 8.02 grams of sulfur?
Answers
Answer:
1.503 x 10^23
Explanation:
your question was how many atoms, so you have to multiply that number which is how many molecules by 8 atoms per molecule of sulphur. So you get 1.503 x 10^23 atoms in 8 grams of sulfur.
hope this helps. please mark me brainliest
if you have 30% of 100 milliliters of an aqueous solution of ethanol, what volumes of ethanol and water are in the solution?
Answers
Volumes of ethanol and water are in the solution : 30 mL and 70 ml
Further explanationThe concentration of a substance can be expressed in several quantities such as moles, percent (%) weight/volume,), molarity, molality, parts per million (ppm) or mole fraction. The concentration shows the amount of solute in a unit of the amount of solvent.
Percent Volume (% v/v) : volume (ml) of solute/100 ml of solution ⇒ ratio of volume of the solute to total volume of the solution
\(\tt \%solute=\dfrac{solute~volume}{solution~volume}\times 100\%\).
30% ethanol ⇒30% etanol, 70% water
In 100 mL solution :
\(\tt Ethanol=30\%\times 100~mL=30~mL\\\\Water=70\%\times 100~mL=70~ml\)
Oxygen atoms (O (g), NOT O2 (g)) react with water vapor to produce hydroxyl radicals (•OH (g)). If water vapor is consumed at a rate of 1.60 x 10^-10 M/s, determine the rate of consumption of O (g).

Answers
The rate of consumption of O (g) if water vapor is consumed at a rate of 1.60 x 10⁻¹⁰ M/s is 8.0 * 10⁻⁹ M/s.
What is the rate of a reaction?The rate of a chemical reaction is known as the reaction rate or rate of reaction. It is proportional to the rise in product concentration per unit of time and the fall in reactant concentration per unit of time.
Considering the given equation:
The equation of the reaction is: O (g) + H₂O (g) ---> 2 •OH (g)
The rate of the consumption of water vapor will be twice the rate of consumption of O (g).
Water vapor is consumed at a rate of 1.60 x 10⁻¹⁰ M/s.
Hence, O (g) will be consumed at a rate of 0.5 * 1.60 x 10⁻¹⁰ M/s = 8.0 * 10⁻⁹ M/s.
Learn more about the rate of a reaction at: https://brainly.com/question/12904152
#SPJ1
What is the relationship between the kinetic energy of an object and the mass of an object? Assume the speed is constant.
A. Kinetic energy gets bigger at the same rate as the mass of an object.
B. Kinetic energy gets bigger at a faster rate than the increase of a mass of an object.
C. Kinetic energy decreases as the mass of an object increases.
D. Kinetic energy decreases at a faster rate than the increase of a mass of an object
Answers
Answer:
A
Explanation:
kinetic and mass are directly proportional
so if one increases the other does to
The relationship between the kinetic energy of an object and the mass of an object is Kinetic energy gets bigger at the same rate as the mass of an object.
What is kinetic energy ?The energy that an object has as a result of motion is known as kinetic energy. It is described as the effort required to move a mass-determined body from rest to the indicated velocity. The body keeps the kinetic energy it acquired throughout its acceleration unless its speed changes.
Potential energy can be moved into motion by a variety of catalysts, including gravity and chemical reactions, to release kinetic energy. As a result, kinetic energy rises and potential energy falls. Mechanical energy is the sum of all kinetic and potential energy.
The capacity to perform work is arguably the most significant characteristic of kinetic energy. Force acting on an object while it is moving is referred to as work. Energy and work are interchangeable because of their tight relationship.
Thus, option A is correct.
To learn more about kinetic energy, follow the link;
https://brainly.com/question/15764612
#SPJ2
Which of the following is an extensive property of water?
Answers
The option that is an extensive property of water is volume. That is option B.
What is an extensive property of water?An extensive property of water is defined as those properties that depends on the amount of water that is taken into consideration.
For example, Volume can be said to be an extensive property of water because the amount of water in the bucket is different from the amount of water in a tablespoon.
Learn more about water here:
https://brainly.com/question/29291605
#SPJ6
Complete question:
Which of the following is an extensive property of water?
a.) temperature
b.) volume
c.) refractive index
d.) Viscosity
If 27.1 grams of bromine and 12.0 grams of chlorine combine to form bromine monochloride, how many grams of bromine monochloride must form
Answers
The amount in grams of bromine monochloride must form is 37.9 grams
To solve the problem, we have to determine which element is the limiting reactant, and calculate how much product is produced based on the amount of the limiting reactant. The balanced chemical equation is as follows:
Br₂ + Cl₂ ⟶ 2BrCl
The molecular weight of Br₂ is 159.808 g/mol. The molecular weight of Cl₂ is 70.90 g/mol. The molecular weight of BrCl is 112.36 g/mol.
The molar masses of the reactants and products are:
Bromine (Br₂) = 27.1 g / 159.808 g/mol = 0.1698 mol
Chlorine (Cl₂) = 12.0 g / 70.90 g/mol = 0.1690 mol
Bromine Monochloride (BrCl) = ?
We can see from the balanced chemical equation that 1 mole of Br₂ reacts with 1 mole of Cl₂ to produce 2 moles of BrCl. The limiting reactant will be the one that has the smallest number of moles. In this case, that is Cl₂. Therefore, Cl₂ is the limiting reactant.
Because 1 mole of Cl₂ reacts with 1 mole of Br₂, we need 0.1690 mol of Br₂ to react with 0.1690 mol of Cl₂.
Using the mole ratio of 1:2, we can determine the amount of BrCl produced:
0.1690 mol Cl₂ x (2 mol BrCl / 1 mol Cl₂) x (112.36 g / 1 mol) = 37.9 g BrCl
Therefore, 37.9 grams of bromine monochloride must form.
Learn more about limiting reactant here: https://brainly.com/question/26905271
#SPJ11
Carry out the following conversions.
(a) 1.21 light-years to miles
Answers
Answer:
About 5.8 trillion miles is equal to 1 light year
so (5,878,600,000,000 miles)*(1.21 light-years) = 7,113,106,000,000 miles
A nitric acid solution (HNO3) has a molar concentration of 0.00044 M. Calculate the [H3O+],[OH-] and the pH of the solution. Remember that Kw=1.0*10^-14M^2
Answers
[H₃O⁺]=4.4 x 10⁻⁴
pH=3.357
[OH⁻] = 2.28 x 10⁻¹¹
Further explanationGiven
HNO3 concentration = 0.00044 M
Required
[H3O+],[OH-] and the pH
Solution
HNO₃ = strong acid
HNO₃ ⇒ H₃O⁺ + NO₃⁻
[H₃O⁺]=[HNO₃]=4.4 x 10⁻⁴
For strong acid pH=-log[H₃O⁺]
pH=-log 4.4 x 10⁻⁴ = 4-log 4.4 =3.357
pOH+pH=pKw
pOH+3.357=14
pOH=14-3.357=10.643
pOH=-log[OH⁻]
\(\tt [OH^-]=10^{-10.643}=2.28\times 10^{-11}\)
What is the chemical formula of the process photosynthesis?
Answers
Answer: Carbon dioxiode+water+sunlight=glucose+oxygen
6CO2 + 6H2O + sunlight —> C6H12O6 + 6O2
Explanation:
what is decomposition reaction
example
Answers
A decomposition reaction is a type of chemical reaction where a compound breaks down into two or more simpler substances. This process is typically induced by heat, light, or an electrical current.
In a decomposition reaction, the reactant compound typically breaks down into two or more products, which can be elements or simpler compounds.
There are various types of decomposition reactions, such as thermal decomposition, electrolytic decomposition, photolytic decomposition, and catalytic decomposition, depending on the type of energy that is used to initiate the reaction.
For example, the decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen (O2) is a decomposition reaction:
\(2H_2O_2 --- > 2H_2O + O_2\)
Thus, this is decomposition reaction.
For more details regarding decomposition reaction, visit:
https://brainly.com/question/16987748
#SPJ1
\( \huge \red {Answer} \)
A decomposition reaction is a type of chemical reaction where a compound breaks down into two or more simpler substances. This process is typically induced by heat, light, or an electrical current.In a decomposition reaction, the reactant compound typically breaks down into two or more products, which can be elements or simpler compounds.How many eggs are in 3 dozen eggs? Use the dimensional analysis for this for practice.
Answers
Answer:
36 eggs
Explanation:
12 eggs in each carton
12 + 12 + 12 = 36 eggs
or 3 * 12 = 36 eggs
Hope this helps, have a nice day/night! :D
What is the name of the compound with the chemical formula Br2F8?
Answers
The name of the compound with the chemical formula Br2F8 is dibromo octafluorine.
How chemical compounds are named?The chemical formula of a compound shows the type and proportion of each element that makes up the chemical compound.
According to this question, a compound with chemical formula Br2F8 is given. The compound consists of the following:
2 atoms of Bromine8 atoms of fluorineTherefore, the name of the compound with the chemical formula Br2F8 is dibromo octafluorine.
Learn more about nomenclature at: https://brainly.com/question/9837065?
A cube measuring 1cm x 1cm x 1cm is full of water, What is the mass of the water in the cube? (Water has a density of 1.0)
Answers
d= m/v
1.0=m/1cm^3
1.0×1cm^3=m
m=1kg/cm^3