Answers
Answer:
yes
Explanation:
volcanoes have done wonderful things for the earth
They help cool off the earth removing heat from its interiorvolcanic emissions have produced the atmosphere and the water of the oceansvolcanoes make islands and add to the continentvolcanoes are necessary because:
fertile is another advantage of volcanic activity."In places like Japan,Indonesia,Philippines,Hawaii,volcanic material mixed in with the soil provides a lot of important nutrients
Related Questions
what is chemical reaction?
Answers
Answer:
a process that involves rearrangement of the molecular or ionic structure of a substance, as distinct from a change in physical form or a nuclear reaction
What is the resource population of the weebugs?
Answers
Insects from the genus Cimex known as bed bugs feast on blood, typically at night and skin rashes.
Thus, Their bites can have a variety of negative health repercussions, such as skin rashes, emotional effects, and allergy symptoms.
The effects of bed bug bites on the skin might range from little redness to obvious blisters. Itching is typically prevalent, and symptoms might take anywhere from minutes to days to manifest.
Some people might experience fatigue or a fever. Usually, impacted bodily parts are those that are exposed. There is no known contagious disease that their bites can spread.Vasculitis and regions of dead skin are unusual complications.
Thus, Insects from the genus Cimex known as bed bugs feast on blood, typically at night and skin rashes.
Learn more about Weedbugs, refer to the link:
https://brainly.com/question/21683913
#SPJ1
which element has the electrons configuration 1s22s22p63s23p64s23d104p2
Answers
The element with the electron configuration 1s22s22p63s23p64s23d104p2 is Silicon (Si).
Explanation:
The electron configuration of an element describes the arrangement of electrons in its atoms. The numbers and letters in the configuration represent the energy levels (n), sublevels (s, p, d, f), and the number of electrons in each sublevel.
In this case, the electron configuration of the element is:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Breaking this down, we can see that the element has:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 6 electrons in the 2p sublevel
- 2 electrons in the 3s sublevel
- 6 electrons in the 3p sublevel
- 2 electrons in the 4s sublevel
- 10 electrons in the 3d sublevel
- 2 electrons in the 4p sublevel
Based on the number of electrons in the outermost energy level (valence electrons), we can determine that this element is in group 14 of the periodic table. Looking at the periodic table, we can see that the
Magma forms within the mantle as a result of _______ temperature and _______ pressure.
All rocks are made of ___________
Answers
Answer:
extreme/high
low
minerals
Explanation:
When Magma forms within the mantle what is the most often result? The most often result of this is high temperatures, and low pressure.
Which of the following is NOT a characteristic of protons?
SELECT AN ANSWER
O Protons are located in the nucleus of an atom.
O A proton has about the same size mass as an electron.
O A proton is a tiny, dense region at the center of the
atom.
Protons have a charge of +1.
Answers
Answer: B
Explanation:
Protons are similar in aize to neutrons. they are larger than electrons.
Rectangular cube 3.2 m length 1.2 m in height and 5 m in length is split into two parts. The container has a movable airtight divider that divides its length as necessary. Part A has 58 moles of gas and part B has 165 moles of a gas.
Required:
At what length will the divider to equilibrium?
Answers
Answer:
The length the divider is to equilibrium from Part A = 1.30 m and from Part B = 3.70 m
Explanation:
Given that:
A rectangular cube with 3.2 m breadth, 1.2 m height and 5 m in length is splitted into two parts.
The diagrammatic expression for the above statement can be found in the attached diagram below.
The container has a movable airtight divider that divides its length as necessary.
Part A has 58 moles of gas
Part B has 165 moles of a gas.
Thus, the movable airtight divider will stop at a length where the pressure on it is equal on both sides.
i.e
\(\mathtt{P = P_A = P_B}\)
Using the ideal gas equation,
PV = nRT
where, P,R,and T are constant.
Then :
\(\mathsf{\dfrac{V_A}{n_A}= \dfrac{V_B}{n_B}}\)
\(\mathsf{\dfrac{L_A \times B \times H}{n_A}= \dfrac{L_B \times B \times H}{n_B}}\) --- (1)
since Volume of a cube = L × B × H
From the question; the L = 5m
i,e
\(\mathsf{L_A +L_B}\) = 5
\(\mathsf{L_A = 5 - L_B}\)
From equation (1) , we divide both sides by (B × H)
Then :
\(\mathsf{\dfrac{L_A }{n_A}= \dfrac{L_B }{n_B}}\)
\(\mathsf{\dfrac{5-L_B}{58}= \dfrac{L_B }{165}}\)
By cross multiplying; we have:
165 ( 5 - \(\mathsf{L_B}\) ) = 58 (
825 - 165\(\mathsf{L_B}\) = 58
825 = 165\(\mathsf{L_B}\) +58
825 = 223\(\mathsf{L_B}\)
\(\mathsf{L_B}\) = 825/223
\(\mathsf{L_B}\) = 3.70 m
\(\mathsf{L_A = 5 - L_B}\)
\(\mathsf{L_A = 5 - 3.70}\)
\(\mathsf{ L_A}\) = 1.30 m
The length the divider is to equilibrium from Part A = 1.30 m and from Part B = 3.70 m

Material use make car tyres and chewing gum?

Answers
Answer:
Rubber
Rubber
Make me brainlyist
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
An 21g sample of platinum metal increase in temperature by 4.90°C when 13.70J of heat are added. What is the specific heat
of platinum?
Answers
Answer:
0.136 J/(g°C)
Explanation:
We can use the formula for specific heat:
q = mcΔT
where q is the amount of heat added, m is the mass of the substance, c is the specific heat, and ΔT is the change in temperature.
Rearranging the formula, we get:
c = q / (m * ΔT)
Substituting the given values, we get:
c = 13.70 J / (21 g * 4.90°C)
c = 0.136 J/(g°C)
Therefore, the specific heat of platinum is 0.136 J/(g°C).
ALLEN
Consider the neutralization reaction
2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq)
A 0.105 L sample of an unknown HNO3 solution required 41.1 mL
of 0.150 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3 solution?
Concentration:
Answers
The concentration of the HNO3 solution is 0.117 M.
To solve this problem, we can use the balanced chemical equation to determine the mole ratio of HNO3 to Ba(OH)2:
2HNO3(aq) + Ba(OH)2(aq) ⟶ 2H2O(l) + Ba(NO3)2(aq)
From the equation, we see that 2 moles of HNO3 react with 1 mole of Ba(OH)2 to produce 2 moles of H2O and 1 mole of Ba(NO3)2. Therefore, the moles of HNO3 in the unknown solution can be calculated from the volume and concentration of Ba(OH)2 used:
moles of Ba(OH)2 = concentration × volume = 0.150 M × 0.0411 L = 0.006165 mo
moles of HNO3 = 2 × moles of Ba(OH)2 = 2 × 0.006165 mol = 0.01233 mol
Finally, we can calculate the concentration of the HNO3 solution:
concentration = moles / volume = 0.01233 mol / 0.105 L = 0.117 M
For more question on concentration click on
https://brainly.com/question/26255204
#SPJ11
Tech A says that the PCM monitors the pre-cat and post-cat oxygen sensors to determine catalytic converter efficiency. Tech B says that a catalytic converter can be tested by graphing the oxygen sensor readings on a scan tool or lab scope and comparing them. Who is correct
Answers
Answer:
Both Tech A and Tech B.
Explanation:
Catalyst is an element used to start chemical reaction but is not used in the reaction. Catalysts material used in catalytic converter include Rhodium, Palladium and platinum. The pre cat and post cat oxygen sensors helps determine converter efficiency.
Answer:
Explanation:
B
Be sure to answer all parts.
The compound 2,3−dimercaptopropanol (HSCH2CHSHCH2OH), commonly known as British anti-Lewisite (BAL), was developed during World War I as an antidote to arsenic-containing poison gas.
(a) If each BAL molecule binds one arsenic (As) atom, how many As atoms can be removed by 1.3 g of BAL?
Enter your answer in scientific notation.
(b) BAL can also be used to remove poisonous heavy metals like mercury (Hg) and lead (Pb). If each BAL binds one Hg atom, calculate the mass percent of Hg in a BAL-Hg complex. (An H atom is removed when a BAL molecule binds an Hg atom.)
Answers
If each BAL molecule binds one arsenic (As) atom, 1.4 x 10^ 22 atoms can be removed by 1.3 g of BAL. BAL can also be used to remove poisonous heavy metals like mercury (Hg) and lead (Pb). If each BAL binds one Hg atom, the mass percent of Hg in a BAL-Hg complex is 61.95 %.
What is mass percent ?The term mass percent of a solution is defined as the ratio of the mass of solute that is present in a solution, relative to the mass of the solution, as a whole.
A. Molar mass of BAL ( C3H8OS2) is 124.2 g/mol
Mass = 2.9 g
Number of moles of BAL = mass / Molar mass
= ( 2.9g / 124.2 g/mol)
= 0.02335 mol
Number of BAL molecules = moles of BAL x Avogadro number
= 0.02335 mol x 6.022 x 10^ 23
= 1.4 x 10^ 22
Then,each BAL binds with 1 As atom. Number o As atoms that can be removed by binding
= 1.4 x 10^ 22
B. BAL molecular formula is C3H8OS2 , so when 1 H si removed and Hg is bonded we get the new formula as C3H7OS2Hg
Molar mass of complex = ( 3 x C atomic mass) + ( 7 x H atomic mass) + O atomic mass + ( 2 x S atomic mass ) + Hg atomic mass
= ( 3 x 12 amu) + ( 7 x 1 amu) + 16 amu + ( 2 x 32 amu) + ( 200.6 amu)
= 323.8 amu
Mass percent of Hg in complex
= 100 x Hg atomic mass / complex molar mass
= ( 100 x 200.6 amu) / ( 323.8 amu)
= 61.95 %
Thus, the mass percent of Hg in a BAL-Hg complex is 61.95 %.
To learn more about the mass percent, follow the link;
https://brainly.com/question/5394922
#SPJ1
Please look at photo mole to mole ratio of each reactant product

Answers
ANSWER
The mass of AlCl3 is 1250.86 grams
The mass of LiH is 298.30785 grams
EXPLANATION
Given reaction
\(\text{ 4LiH + AlCl}_3\text{ }\rightarrow\text{ LiAlH}_4+\text{ 3LiCl}\)The mass of LiAlH4 is 356 grams
To find the mass of the reactants, follow the steps below
Step 1: Find the number of moles of LiAlH4 using the below formula
\(\text{ Mole = }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, that the molar mass of LiAlH4 is 37.95 g/mol as provided in the periodic table
\(\begin{gathered} \text{ Mole = }\frac{\text{ mass}}{\text{ molar mass}} \\ \text{ Mole = }\frac{356}{37.95} \\ \text{ Mole = 9.381 moles} \end{gathered}\)Hence, the number of moles of LiAlH4 is 9.381 moles
Step 2: Find the number of moles of LiH and AlCl3 using a stoichiometry ratio
From the reaction, 4 moles of LiH give 1 mole of LiAlH4
Let x represents the number of moles of LiH
\(\begin{gathered} 4\text{ }\rightarrow\text{ 1} \\ x\text{ }\rightarrow\text{ 9.381} \\ \text{ cross multiply} \\ x\times1\text{ = 4 }\times\text{ 9.381} \\ x\text{ = 37.523 moles} \end{gathered}\)The number of moles of 37.523 moles
Find the number of moles of AlCl3?
1 mole of AlCl3 gives 1 mole LiAlH4
Let x represents the number of moles of AlCl3
\(\begin{gathered} 1\text{ }\rightarrow\text{ 1} \\ x\text{ }\rightarrow9.381 \\ cross\text{ mulitiply} \\ 1\text{ }\times\text{ 9.381 = x }\times1 \\ 9.381\text{ = x} \\ x\text{ = 9.381 moles} \end{gathered}\)The number of moles of AlCl3 is 9.381 moles
Step 3: Find the mass of LiH and AlCl3 using the below formula
\(\text{ Mole= }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, that the molar mass of LiH is 7.95 g/mol, and the molar mass of AlCl3 is 133.34 g/mol as provided in the provided table.
For LiH
\(\begin{gathered} \text{ Mole = }\frac{mass}{molar\text{ mass}} \\ \text{ mole = 37.523 moles, and molar mass = 7.95 g/mol} \\ \text{ 37.523 = }\frac{mass}{7.95} \\ mass\text{ = 37.523 }\times\text{ 7.95} \\ \text{ mass = 298.30785 grams} \end{gathered}\)Hence, the mass of LiH is 298.30785 grams
For AlCl3
\(\begin{gathered} \text{ Mole = }\frac{mass}{molar\text{ mass}} \\ \text{ mole = 9.381 moles, molar mass = 133.34 g/mol} \\ \text{ 9.381 = }\frac{mass}{133.34} \\ \text{ cross multiply} \\ \text{ Mass = 9.381}\times133.34 \\ \text{ Mass = 1250.86 grams} \end{gathered}\)The mass of AlCl3 is 1250.86 grams
Predict the chemical shifts for the signals in the proton NMR spectrum of each of the following compounds.

Answers
The proximity of unsaturated groups (C=C, C=O, aromatic) and electronegative atoms (O, N, halogen) has an impact on the proton NMR chemical shift. Electronegative groups shift to the left (down field; ppm rise).
What does NMR spectroscopy's chemical shift entail?The chemical shift in nuclear magnetic resonance (NMR) spectroscopy refers to the atomic nucleus' resonant frequency in relation to a standard in a magnetic field. The location and quantity of chemical changes frequently serve as diagnostic indicators of molecular structure.
You take into account the chemically non-equivalent proton(s) one at a time while making chemical shift predictions. Find the origin of each proton or proton pair that is not chemically comparable. Whether the proton(s) is/are linked to a methyl, methene, or methine determines the beginning point.
learn more about nuclear magnetic resonance
https://brainly.com/question/21024524
#SPJ1
Can somebody please help me understand this? I don't understand what I need to do to solve any of the parts.

Answers
This technique has been used to identify the presence of gases such as oxygen, methane, and carbon dioxide in the atmospheres of exoplanets.
i) To estimate the frequency of the violet (leftmost) emission, we can use the equation v = c/λ, where v is frequency, c is the speed of light (3.00 x 10^8 m/s), and λ is the wavelength of the emission in meters. The wavelength of the violet emission is 400 nm or 400 x 10^-9 m, so the frequency can be calculated as v = (3.00 x 10^8 m/s) / (400 x 10^-9 m) = 7.50 x 10^14 Hz.
ii) To estimate the energy of the violet emission, we can use the equation E = hv, where E is energy, h is Planck's constant (6.63 x 10^-34 Js), and v is frequency in Hz. Substituting the frequency calculated in part (i), we get E = (6.63 x 10^-34 Js) x (7.50 x 10^14 Hz) = 4.97 x 10^-19 J.
b. The spectral lines are produced by the electrons within the atoms of this element, which can absorb or emit specific amounts of energy to move between different energy levels. These energy transitions result in the emission or absorption of photons with specific wavelengths and frequencies, giving rise to the observed emission spectrum.
c. The violet emission line represents the photon with the most energy since it has the shortest wavelength (400 nm) and highest frequency (7.50 x 10^14 Hz) among the lines shown. This highest energy does not necessarily represent the energy of the valence electrons, but rather corresponds to the specific energy transitions occurring within the atoms of the element.
d. Emission spectra can be used to determine the gases present in the atmosphere of a far-away planet by analyzing the specific wavelengths of the emitted or absorbed light from the planet. Each gas has a unique emission or absorption spectrum, allowing scientists to identify the gases present in the planet's atmosphere.
To know more about Wavelength , visit :
https://brainly.com/question/13533093
#SPJ1
How many particles are there in 0.057 moles of lithium bromide made
Answers
There are 3.44 x 10^{22} particles in 0.057 moles of lithium bromide.
What chemical compound is lithium bromide known by?The lithium bromide formula also known as the lithium monobromide formula or Bromo lithium formula is explored. It is a counterion bromide-based salt of lithium.
we have to use Avogadro's constant,
Avogadro's constant, is approximately equal to 6.022 x 10^{22} particles per mole.
we can use the following formula:
number of particles = moles x Avogadro's constant
Substitute the values,
number of particles = 0.057 moles x 6.022 x 10^{23} particles/mol
Simplifying the equation
number of particles = 3.44 x 10^{22} particles
To know more about lithium bromide visit:
https://brainly.com/question/16584013
#SPJ9
Refer to the following table of Ksp values to answer the question below.
Compound
PbS
SnS
ZnS
HgS
Ksp
3 x 10-29
1 x 10-26
2x 10-25
1.6 x 10-52
A solution has 0.01 M Pb²+, 0.01 M Sn²+, 0.01 M Zn²+, and 0.01 M Hg2+. As tiny amounts of sulfide ion are slowly added
to the solution, which compound will precipitate first?
PbS
O Fes
HgS
SnS
Answers
HgS will precipitate first when tiny amounts of sulfide ion are slowly added to the solution.
What is Ksp?The solubility product constant, Ksp, is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution.
If Solubility product is greater than the ionic product then no precipitate will form on adding more solute because unsaturated solution is formed.
If Solubility product is lower than the ionic product then excess solute will precipitate out because of the formation of super saturated solution.
Here, Ksp value of HgS is very low. Hence, HgS will precipitate first when tiny amounts of sulfide ion are slowly added to the solution.
Learn more about Ksp here:
https://brainly.com/question/4637627
#SPJ1
By placing this passage at the beginning of the story, the author builds suspense for the arrival of
.
Answers
How much energy is required to heat up a Styrofoam cup from 25oC to 40oC? The mass of the cup is 20 grams and the specific heat capacity of Styrofoam is 1.1 J/(g *oC).
Answers
Answer:
Q = 440 J
Explanation:
Given data:
Heat required = ?
Initial temperature = 25°C
Final temperature = 40°C
Mass in gram = 20 g
Specific heat capacity = 1.1 J/g.°C
Solution:
Formula:
Q = m.c. ΔT
Q = amount of heat absorbed or released
m = mass of given substance
c = specific heat capacity of substance
ΔT = change in temperature
ΔT = 40°C - 20°C
ΔT = 20°C
Q = 20 g×1.1 J/g.°C × 20°C
Q = 440 J
17. HAZWOPER training and certification recognizes:
a. A large number (as much as 80%) will self-present or be self-referred victims
b. Awareness level training will promote proper initial triage actions
c.
Victims will use any entrance they can enter at the hospital, in addition to the
emergency department entrance
d. Both A and C
Answers
HAZWOPER training and certification recognize:
a large number (as much as 80%) will self-present or be self-referred victimsVictims will use any entrance they can enter at the hospital, in addition to the emergency department entranceThe correct option is both A and C
What is the HAZWOPER training and certification?HAZWOPER (Hazardous Waste Operations and Emergency Response) training and certification recognize that a large number of victims (as much as 80%) in hazardous waste incidents or emergencies will self-present or be self-referred for medical treatment.
Additionally, HAZWOPER training acknowledges that victims may use any entrance they can access at a hospital, not just the emergency department entrance.
This is because individuals affected by hazardous materials may arrive at different areas of the hospital seeking medical assistance.
Therefore, option d. Both A and C are correct statements regarding the recognition of HAZWOPER training and certification.
Learn more about HAZWOPER at: https://brainly.com/question/31561828
#SPJ1
What is the purpose of the arrow in a chemical equation?
Answers
The arrow in a chemical equation represents the direction of the reaction. It indicates the conversion of reactants into products. The arrow points from the reactant side to the product side, symbolizing the flow of the reaction.
The purpose of the arrow is to visually represent the chemical transformation occurring in the reaction. It shows the relationship between the reactants and products and the direction in which the reaction proceeds. The arrow implies that the reactant molecules are being rearranged and transformed into new substances with different properties.
Chemical equations are used to describe the stoichiometry and balance of reactions. The arrow helps convey this information by illustrating the overall process taking place. It serves as a crucial element in understanding the reaction's composition, reaction conditions, and the substances involved.
Furthermore, the arrow also implies that the reaction can occur in both directions. In reversible reactions, the arrow can be represented as a double-headed arrow, indicating that the reaction can proceed in either direction depending on the conditions.
Know more about reversible reactions here:
https://brainly.com/question/21426719
#SPJ8
Aretha measures a circuit at 110 V and 240 . Using Ohm’s law, what can she calculate for the circuit?
voltage
ohms
resistance
current
Answers
Answer:
C 100% correct. trust me
Explanation:
Only when the given temperature and the other physical variables remain constant does Ohm's law apply. Increasing the current causes the temperature to rise in some components. Here the voltage is 110 V and the current is 240, so the resistance can be measured. The correct option is C.
What is Ohm's law?The relationship between electric current and potential difference is stated by Ohm's law. Under the assumption that all physical parameters and temperatures remain constant, Ohm's law asserts that the voltage across a conductor is directly proportional to the current flowing through it.
Most conductors' current is directly proportional to the voltage applied to them. German physicist Georg Simon Ohm was the first to empirically confirm Ohm's law. Ohm's law is one of the most fundamental and significant principles governing electrical circuits.
The equation is V = IR, 'R' is the resistance and 'I' is the current and 'V' is the voltage.
Thus the correct option is C.
To know more about Ohm's law, visit;
https://brainly.com/question/14796314
#SPJ5
Why do you think Petri dishes contain sugar?
Answers
Answer:
sugar is mainly responsible for growth of yeast, but in BG11 medium there is no sugar
Explanation:
Although petri dishes do not contain sugar by themselves, the growth media used in these dishes can contain sugar to offer an energy source for the microorganisms being grown.
In laboratories, Petri plates are often used to cultivate and develop microorganisms such as bacteria, fungus, and other tiny creatures. Dishes are typically constructed of glass or clear plastic and have a flat, shallow, cylindrical shape with a cover. Petri plates do not contain sugar by definition; rather, they are utilised as containers for many types of growth media, including ones containing sugar.
To know more about petri dishes, here:
https://brainly.com/question/28796560
#SPJ3
Which statements best support the student’s claim? Select two of the five statements.

Answers
The statements that best support the student’s claim is that
The cells contains rigid structures that supports and protects plant cells.The cells contains structures that converts light energy to chemical energy.What is a cell?A cell is described as the smallest unit that can live on its own and that makes up all living organisms and the tissues of the body.
A cell has three main parts which include :
the cell membrane, the nucleus, and the cytoplasm.The cell membrane surrounds the cell and controls the substances that go into and out of the cell.
In conclusion, Cells are the basic building blocks of all living things.
Learn more about cells at:
https://brainly.com/question/13920046
#SPJ1
During the process of _______________ the solute maybe completely ionized, partially ionized, or it may remain intact.
Answers
Answer:
Dissolution.
Explanation:
In Chemistry, dissolution can be defined as the process of dissolving or dissociating a solute in solid, liquid or gaseous phase into fragmented particles by a solvent in order to form a solution. For dissolution to occur in solids, the crystalline structure of the substance must be broken up so as to release ions, atoms or molecules to produce a solution. For liquid and gases, the substance to be dissolved must form a non-covalent bond with the solvent to produce a solution.
Hence, during the process of dissolution the solute maybe completely ionized, partially ionized, or it may remain intact.
Solubility is a term used to describe how readily a substance can be dissolved in a solvent to form a solution. Thus, a substance is said to be soluble if it dissolves completely in a solvent and insoluble if it doesn't dissolve or only dissolves partially.
For example, salt is said to be soluble because it dissolves completely in water. The sodium chloride (NaCl) when mixed with water dissociates into sodium and chloride ions.
Please Help me solve for B
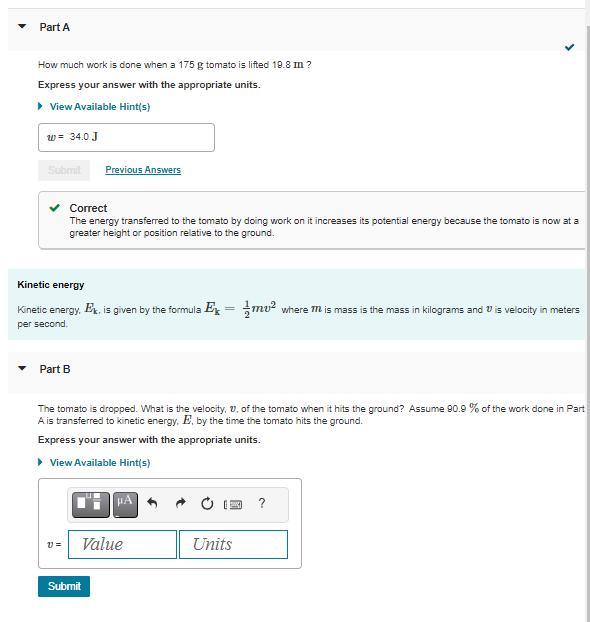
Answers
The velocity of the tomato when it hits the ground is approximately 13.49 meters per second.
The potential energy of the tomato is at the height of 10 meters. When the tomato hits the ground, most of the potential energy is E1 = 0.909*mgh.
By the conservation of energy principle, the kinetic energy \(E_1\) is equal to the kinetic energy \(E_2\) of the tomato just before it hits the ground.
The kinetic energy \(E_2\) is given by\(1/2mv^2\), where v is the velocity of the tomato just before it hits the ground. Equating \(E_1\) and \(E_2\) solving for v, we get:
\(v = \sqrt{(20.909gh)\)
Substituting the values of \(g = 9.81 m/s^2\)and h = 10 m, we get:
v = \(\sqrt{(20.9099.81*10)}\) = 13.49 m/s
To know more about potential energy, here
brainly.com/question/24284560
#SPJ1
--The complete Question is, Suppose a tomato is dropped from a height of 10 meters. If 90.9% of the work done on the tomato is converted to kinetic energy by the time it hits the ground, what is the velocity (in meters per second) of the tomato when it hits the ground? --
Write a net ionic equation to show that triethylamine, (C2H5)3N, behaves as a Bronsted-Lowry base in water.
Answers
Answer:
\((C_2H_5)_3N~+~H_2O~->~(C_2H_5)_3NH^+~+~OH^-\)
Explanation:
For this question, we have to remember that definition of acid and base in the Bronsted-Lowry theory:
Acid
A substance with the ability to produce a hydronium ion (\(H^+\)).
\(HA~->~H^+~+~A^-\)
Base
A substance with the ability to accepts a hydronium ion (\(H^+\)).
\(B~+~H^+->BH^+\)
If we check the reaction mechanism (figure 1). We can see that the lone pair of electrons in the "N" atom will remove an "H" from the water molecule producing a positive charge in the nitrogen and a hydroxyl group (\(OH^-\)).
With all this in mind, the net ionic equation would be:
\((C_2H_5)_3N~+~H_2O~->~(C_2H_5)_3NH^+~+~OH^-\)
I hope it helps!

I AM GIVING A LOT OF POINTS SO PLEASE HELP ME!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Can someone please help me with this?
Rules:
Label the section of the roller coaster where the kinetic or potential energy is.

Answers
Answer:c
Explanation:
it’s gained kinetic from the gravitational potential energy at the top
So, potential energy would be: A
And kinetic energy would be: B, C, D
I’m almost sure these would be correct.
Hope this helps!! (:
Why does the Sun gravitationally dominate all other objects in the Solar System?
Answers
Sometimes a simple incident in our lives can actually have a deeper meaning and impact on us than might be apparent at the time. Write a reflective narrative about a memorable experience in your life.
Answers
I remember the day when I was walking to the grocery store and saw an elderly woman sitting on the sidewalk with her cart. She looked tired and was trying to catch her breath. At first, I walked past her, not wanting to get involved. But then something made me stop and turn around. I approached her and asked if she needed any help. She told me that she had been walking for a while and needed to rest for a bit. I helped her sit down on a nearby bench, and we started talking.
As we talked, I learned that her name was Mary, and she had lived in the neighborhood for over 50 years. She shared stories of her life and her struggles, and I listened with empathy. I could see the gratitude in her eyes, and it made me realize the power of human connection.
That experience taught me that we should never underestimate the impact of a simple act of kindness. It made me more aware of the people around me and more willing to help others in need. It also showed me the importance of empathy and how it can make a difference in someone's life.
Looking back, that moment was a turning point for me. It made me more compassionate and more aware of the needs of others. It showedme that a small gesture of kindness can have a ripple effect and make a big difference in someone's life. It also reminded me that we all have our own stories and struggles, and sometimes all we need is someone to listen and show us a little empathy.
Since that day, I have made it a point to be more aware of the people around me and to offer help whenever I can. Whether it's holding the door open for someone or offering a listening ear, I try to be more present and empathetic in my interactions with others.
That experience also taught me the importance of slowing down and taking the time to connect with people. In our fast-paced world, it's easy to get caught up in our own lives and forget about those around us. But by taking the time to connect with others, we can create meaningful relationships and make a positive impact in the world.
Overall, that simple encounter with Mary taught me a valuable lesson about the power of empathy and human connection. It's a lesson that I will always carry with me and strive to live by in my daily life.
In English, you're asked to write a reflective narrative about a life event with a deeper impact than initially visible. Describe the event, your reaction, its deeper meaning, and how it shaped you.
Explanation:This question pertains to reflective writing, a style typically used in English subject coursework. The task is about developing a narrative based on a memorable experience in your life that had a deeper impact than initially visible. Begin by selecting an event, which while appearing simple, had profound implications in retrospect. Describe the event in detail (who, what, where, when), discussing your personal reaction at the time. Then, explain its deeper meaning or the impact it had on you. Drawing conclusions about how this event shaped your perspectives or behaviours would constitute the reflective element of your narrative.
Learn more about Reflective Narrative here:https://brainly.com/question/33018651
#SPJ2