Brass and bronze are both of copper because copper is the component in brass and bronze.
Answers
Answer:1 alloys 2 main
Explanation: Brass and bronze are both constructed from copper because copper is the main component in brass
Related Questions
What volume of H2 is produced at 315 K and 1.25 atm when 3.50 grams of Zn reacts with excess HCl?
Zn(s) + 2 HCl(aq) → H2(g) + ZnCl2(aq)
Answers
The volume of hydrogen gas produced at 315 K and 1.25 atm when 3.50 grams of zinc reacts with excess HCl is 1.16 L.
The balanced chemical equation for the reaction between zinc and hydrochloric acid is:
\(Zn(s) + 2 HCl(aq) → H2(g) + ZnCl2(aq)\)
We need to find the volume of hydrogen gas produced at 315 K and 1.25 atm when 3.50 grams of zinc reacts with excess HCl.
First, we need to determine the number of moles of zinc used in the reaction. The molar mass of zinc is 65.38 g/mol, so:
n(Zn) = 3.50 g / 65.38 g/mol = 0.0536 mol
Since 1 mole of zinc reacts with 1 mole of hydrogen gas, the number of moles of hydrogen gas produced is also 0.0536 mol.
Using the ideal gas law, PV = nRT, we can calculate the volume of hydrogen gas produced. We are given the temperature (315 K) and the pressure (1.25 atm), and the gas constant R is 0.0821 L·atm/(mol·K).
Substituting the values into the equation, we get:
V = (nRT) / P
V = (0.0536 mol) x (0.0821 L·atm/(mol·K)) x (315 K) / (1.25 atm)
V = 1.16 L
Therefore, the volume of hydrogen gas produced at 315 K and 1.25 atm when 3.50 grams of zinc reacts with excess HCl is 1.16 L.
To learn more about hydrogen gas , here
https://brainly.com/question/14692538
#SPJ1
Pt 2. Chem Reactions 50 PTS
Hey, I need help with Chemistry. Please also provide an explanation as well!!



Answers
The red colour is the limiting reactant.
Red-blue colour ball and two white balls attached together are reactants.
Red-blue colour ball and two white and one red colour ball attached to each other are products.
What is a limiting reagent?The reactant that is entirely used up in a reaction is called a limiting reagent.
A reactant is a substance that is present at the start of a chemical reaction. The substance(s) to the right of the arrow are called products.
A product is a substance that is present at the end of a chemical reaction.
Hence,
The red colour is the limiting reactant.
Red-blue colour ball and two white balls attached together are reactants.
Red-blue colour ball and two white and one red colour ball attached to each other are products.
Learn more about limiting reagents here:
brainly.com/question/26905271
#SPJ1
What does the salinity of seawater represent?
The concentration of salt dissolve in the ocean
The concentration of all dissolved chemicals in the ocean
The volume of salt in the ocean
the mass of salt in the ocean
Answers
Answer:
Salinity is a measure of the 'saltiness' of seawater, or more precisely the amount of dissolved matter within seawater.
equal volumes of 0.10-molar h3po4 and 0.20-molar koh are mixed. after equilibrium is established, the type of ion in solution in largest concentration, other than the k ion, is a. H2PO4Â
b. HPO42Â
d. OHÂ
c. PO43Â
e. H3O+
Answers
After equilibrium is established, then type of ion in solution in largest concentration, other than the k ion, is : (b) HPO₄²⁻.
What is meant by equilibrium?In chemistry, equilibrium is a state of balance or stability achieved in chemical reaction when the rates of forward reaction and reverse reaction are equal.
The balanced chemical equation for the reaction between H₃PO₄ and KOH is:
H₃PO₄ + 3KOH → K₃PO₄ + 3H₂O
In this reaction, one mole of H₃PO₄ reacts with three moles of KOH to produce one mole of K₃PO₄ and three moles of water.
When equal volumes of 0.10 M H₃PO₄ and 0.20 M KOH are mixed, the concentration of OH⁻ ions will be in excess because KOH is strong base and H₃PO₄ is a weak acid. The OH⁻ ions will react with H⁺ ions of H₃PO₄ to form water, according to following reactions:
H₃PO₄ + OH⁻ → H₂PO₄⁻ + H₂O
H₂PO₄⁻ + OH⁻ → HPO₄²⁻ +H₂O
The net effect of these reactions is that H₃PO₄ reacts with OH⁻ to produce HPO₄²⁻. Therefore, the type of ion in solution in largest concentration, other than the K+ ion, is HPO₄²⁻.
To know more about equilibrium, refer
https://brainly.com/question/18849238
#SPJ1
A person is exercising at an absolute VO
2
of 0.92 L/min and they weigh 125lbs. What is their relative VO
2
?
Answers
The person's relative VO2 is 16.23 mL/kg/min. Absolute VO2 refers to the total volume of oxygen that the body consumes during exercise. It is typically measured in liters per minute (L/min).Relative VO2, on the other hand, takes into account the individual's body weight. It is typically expressed in milliliters of oxygen per kilogram of body weight per minute (mL/kg/min).
Here's how to calculate relative VO2:
Step 1: Convert the person's weight from pounds to kilograms.1 pound = 0.45 kilograms
Therefore, 125 pounds = 56.7 kilograms
Step 2: Divide absolute VO2 by body weight in kilograms and multiply by 1000 to convert to mL/kg/min
.Relative VO2 = (absolute VO2 ÷ body weight in kg) × 1000Relative VO2 = (0.92 L/min ÷ 56.7 kg) × 1000Relative VO2 = 16.23 mL/kg/min
Therefore, the person's relative VO2 is 16.23 mL/kg/min.
To know more about relative VO2, visit:
https://brainly.com/question/6463365
#SPJ11
Please help
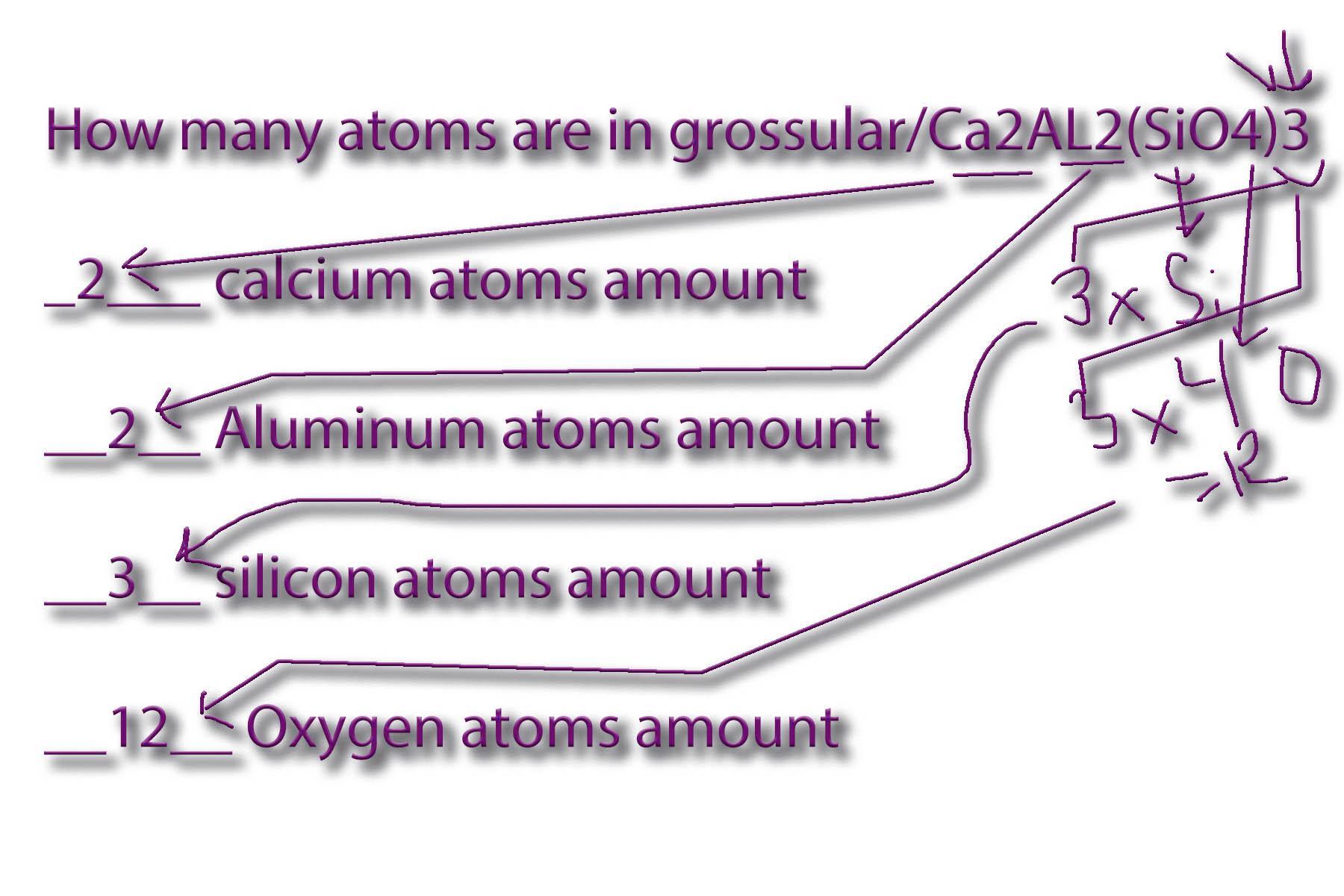
How many atoms are in grossular/Ca2AL2(SiO4)3
____ calcium atoms amount
____ Aluminum atoms amount
____ silicon atoms amount
____ Oxygen atoms amount
Answers
Answer:
See below
Explanation:
How many atoms are in grossular/Ca2AL2(SiO4)3
_2___ calcium atoms amount
__2__ Aluminum atoms amount
__3__ silicon atoms amount
__12__ Oxygen atoms amount
See attached worksheet
What is conduction? (4 points)
a Transfer of heat through circular movement
b Transfer of heat through direct physical contact
c Transfer of heat through electromagnetic waves
d Transfer of heat through the movement of particles
Answers
Answer:
Your Answer would be Option B
Answer:
B
Explanation:
The Answer is B-Transfer of heat through direct physical contact
What is the highest level of complexity for a single celled organism?
Answers
Answer:
Amoeba since it can come together as a multicellular with other amoebas, but still act as one giant living cell.
Explanation: i read in a book that red-slime molds can do that. hope it wasn't too far too late recent.
What is the empirical formula of a compound with a percent composition of 22.5% Phosphorous and 77.5% Chlorine?
Answers
Answer:
\(\boxed {\boxed {\sf PCl_3}}\)
Explanation:
We are given the percent composition: 22.5% phosphorus and 77.5% chlorine.
We can assume there are 100 grams of this compound. We choose 100 because we can simply use the percentages as the masses.
22.5 g P 77.5 g ClNext, convert these masses to moles, using the molar masses found on the Periodic Table.
P: 30.974 g/mol Cl: 35.45 g/molUse the molar masses as ratios and multiply by the number of grams. \(22.5 \ g \ P * \frac {1 \ mol \ P }{30.974 \ g \ P}= \frac {22.5 \ mol \ P }{ 30.974} = 0.7264157035 \ mol \ P\)
\(77.5 \ g \ Cl * \frac {1 \ mol \ Cl }{35.45 \ g \ Cl}= \frac {77.5 \ mol \ Cl }{ 35.45} \ =2.186177715 \ mol \ Cl\)
Divide both of the moles by the smallest number of moles to find the mole ratio.
\(\frac {0.7264157035} {0.7264157035} = 1\)
\(\frac {2.186177715}{0.7264157035}=3.009540824 \approx 3\)
The mole ratio is about 1 P: 3 Cl, so the empirical formula is written as: PCl₃
Convert 31.82 grams of ca(oh)2 to moles Convert 3.2 moles of k2so3 to grams Convert 7.25x10^23 formula units of hcl to moles Convert 46.6L of cl2 gas to moles at stp
Answers
Answer:
See explanation
Explanation:
Number of moles = given mass/molar mass
For Ca(OH)2
n= 31.82g/74g/mol= 0.43 moles
n = mass/ molar mass
Molar mass of K2SO3 = 158g/mol
mass= 3.2 × 158 = 505.6 g
1 formula unit = 6.02 × 10^23
1 mole = 6.02 × 10^23
x moles = 7.25x10^23
x = 7.25x10^23/6.02 × 10^23
x= 1.2 moles
1 mole of Cl2 occupies 22.4 L at STP
x moles of Cl2 occupies 46.6L
x = 46.6/22.4
x= 2.1 moles
Isotopes of the same element vary from each other in their number of:
neutrons
electrons
protons
Answers
Answer:
Neutrons
Explanation:
A particular concentration of a chemical found in polluted water has been found to be lethal to 26% of the fish that are exposed to the concentration for 24 hours. Twenty-nine fish are placed in a tank containing this concentration of chemical in water. (a) Use R to calculate the probability that exactly 20 survive. (Round your answer to three decimal places.) (b) Use R to calculate the probability that at least 14 survive. (Round your answer to three decimal places.) (c) Use R to calculate the probability that at most 23 survive. (Round your answer to three decimal places.) (d) Use R torfind the mean and variance of the number that survive. (Round your variance to two decimal places.) mean variance
Answers
Calculating probabilities in R
(a) The probability that exactly 20 fish survive can be calculated using the binomial probability formula in R. Let's denote this probability as P(X = 20), where X follows a binomial distribution with n = 29 (total number of fish) and p = 0.74 (probability of survival, calculated as 1 - 0.26). The calculation in R would be:
```R
dbinom(20, size = 29, prob = 0.74)
```
The resulting probability is the answer to part (a).
(b) The probability that at least 14 fish survive can be calculated by summing the probabilities of 14, 15, 16, and so on, up to 29. Denoting this probability as P(X ≥ 14), the calculation in R would be:
```R
sum(dbinom(14:29, size = 29, prob = 0.74))
```
The resulting probability is the answer to part (b).
(c) The probability that at most 23 fish survive can be calculated by summing the probabilities of 0, 1, 2, and so on, up to 23. Denoting this probability as P(X ≤ 23), the calculation in R would be:
```R
sum(dbinom(0:23, size = 29, prob = 0.74))
```
The resulting probability is the answer to part (c).
(d) To find the mean and variance of the number of fish that survive, we can use the formulas for the binomial distribution. The mean (μ) is calculated as n * p, and the variance (σ²) is calculated as n * p * (1 - p). Using the given values of n = 29 and p = 0.74, we can calculate the mean and variance in R:
```R
n <- 29
p <- 0.74
mean <- n * p
variance <- n * p * (1 - p)
```
The resulting mean and variance values are the answer to part (d).
What are the probabilities and statistics calculated in R for fish survival in polluted water?
(a) To calculate the probability that exactly 20 fish survive, we use the binomial probability formula in R, which considers the total number of fish (n = 29) and the probability of survival (p = 0.74). The resulting probability represents the likelihood of observing exactly 20 surviving fish.
(b) The probability that at least 14 fish survive is calculated by summing the probabilities of 14, 15, 16, and so on, up to the total number of fish (29). This cumulative probability indicates the likelihood of observing 14 or more surviving fish.
(c) The probability that at most 23 fish survive is calculated by summing the probabilities of 0, 1, 2, and so on, up to 23. This cumulative probability represents the likelihood of observing 23 or fewer surviving fish.
(d) The mean and variance of the number of fish that survive can be found using the formulas for the binomial distribution. The mean (μ) is equal to the product of the total number of fish (n) and the probability of survival (p).
The variance (σ²) is calculated as the product of n, p, and (1 - p). These values provide insights into the average and variability of fish survival in the given conditions.
Learn more about probability
brainly.com/question/31828911
#SPJ11
How is bond polarity determined?
Answers
Answer:
Explanation:
Are you a gril or boy
Explanation:
you use it yo determine the polarity of a covalent bond using numerical means find the difference between the electron gravity of atoms ; if the result is between 0.4 and 1.7 then generally the bond is polar covalent . pls follow and mark brainliantest
Titanium consists of two isotopes of masses 46.90 amu and 48.95 amu. If the first isotope has a percent abundance of 70.84%, what is the percent abundance of the second isotope?
Answers
Answer: 29.16%
Explanation:
The total of 2 percentages need to equal 100% so we subtract the percent they gave us from 100.
assume that all 115.0 g of carbohydrates in the meal are glucose, c6h12o6. how many molecules of acetyl-coa will be produced from glycolysis?
Answers
2 Acetyl CoA molecules are generated after glycolysis.
What is glycolysis ?The metabolic process known as glycolysis transforms glucose (C6H12O6) into pyruvate (CH3COCO2H). The high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide are formed using the free energy released during this process (NADH). A series of ten enzyme-catalyzed processes is known as glycolysis.
A list of the 10 processes involved in the glycolysis pathway. An oxygen-free metabolic pathway is glycolysis (In anaerobic conditions pyruvate is converted to lactic acid). The widespread prevalence of glycolysis in different species suggests that it is an old metabolic system. In fact, the events that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, take place in the oxygen-free environment of the Archean oceans, likewise in the absence of enzymes, and are activated by metal.
To learn more about glycolysis Please click on the given link:
https://brainly.com/question/1966268
#SPJ4
What is required to make an object at rest move
Answers
Answer:
Newton's First Law is the law of inertia. An object with no net forces acting on it which is initially at rest will remain at rest. If it is moving, it will continue to move in a straight line with constant velocity. F is the net force acting on an object.
Explanation:
yay hope this helps.
it need force or something
I need help with this question

Answers
Since 12C’s molar mass is 12 grams, 48 grams of 12C atoms would be equal to _____ moles.
Answers
Since 12C’s molar mass is 12 grams, 48 grams of 12C atoms would be equal to 4 moles.
Carbon, is a chemical element having symbol C and its atomic number 6. Carbon atoms have six protons and usually have six neutrons in their nucleus, although isotopes of carbon with different numbers of neutrons exist.
Since the molar mass of 12C is 12 grams/mole, we can use the following formula to calculate the number of moles;
moles = mass / molar mass
Substituting the given values, we get;
moles = 48 g / 12 g/mol
moles = 4 mol
Therefore, 48 grams of 12C atoms is equal to 4 moles of 12C.
To know more about molar mass here
https://brainly.com/question/12127540
#SPJ1
How many total atoms are in
4Ca(HCO3)2? Sorry I can't do a subscript got the 3 and the 2. Thanks
Answers
Calcium Hydroxide has three atoms 5 atoms in each molecule.
What is chemical formula?The number of atoms in each element of a compound is revealed by the chemical formula. It includes the symbols for the atoms of each element found in the compound, together with a count of how many of each element there are overall in the form of subscripts.One hydrogen atom, one carbon atom, and three oxygen atoms make up the HCO3-one bicarbonate ion.For HCO3 H C O 3, there is a subscript 2 indicating that there will be 2 hydrogen atoms in the chemical. It takes two hydrogen atoms to answer.In order to determine the amount of hydrogen atoms in a certain molecule, we must count the number of different groups, which comes to three. Since each group of syllables has three hydrogen atoms, multiplying three by three results in nine. Therefore, we can write a final response stating that the molecule will have nine hydrogen items.To learn more about chemical formula refer to:
https://brainly.com/question/26388921
#SPJ1
a student completes a titration using 63.42 ml of 3.5 m hcl. how many moles of hcl were used in the titration?
Answers
Molarity or concentration, is the number of moles present in one liter of volume.
Molarity, M = number of moles / Volume in liters
Unit of Molarity is mole/ liter second or the Molar represented by M.
We are given with a case with
Volume = 63.42 ml
Molarity = 3.5 M
and are asked to determine number of moles.
M = number of moles / Volume in liters
number of moles = volume × Molarity
number of moles of HCl = (63.42)(3.5)/1000
number of moles of HCl = .22197 moles
To know more about Molarity:
brainly.com/question/8732513
#SPJ4
A student is given an object and is asked to identify its density. The object has a volume of 3 cubic centimeters and a mass of 6 grams. Which of the following equations correctly sets up the formula for density?
Answers
Density =mass/volume
=6/3
=2
express the answer to each of the following calculations in scientific notation with the correct number of significant figures: 45.0 x 270
Answers
Answer: 1.215 × 10^4 i think
Explanation:
one definition of a base is a substance that provides which ion in water solution?
Answers
A base is a substance that provides hydroxide ions (OH-) in water solution.Therefore, hydroxide ions are a characteristic feature of bases.
Bases are substances that have a pH greater than 7 and can neutralize acids. They react with acids to form salt and water. In water solution, bases dissociate and release hydroxide ions (OH-), which can accept protons (H+) from acids to form water. The more hydroxide ions a substance releases in water, the stronger the base is.
When a base is dissolved in water, it releases hydroxide ions (OH-), which can react with hydrogen ions (H+) from an acid to form water (H2O). This property is what gives bases their characteristic alkaline properties and allows them to neutralize acids.
To know more about hydroxide visit:
https://brainly.com/question/31820869
#SPJ11
Iron will react with water to produce an iron oxide and hydrogen gas. Which equation below represents a correctly balanced equation for this reaction?
A . a
B. b
C. c
D. d

Answers
The balanced equation for the reaction of iron with water that results in the production of iron oxide and hydrogen gas is
3 Fe (s) + 4H₂O (g) → Fe₃O₄ (s) + 4H₂ (g)
Iron does not react directly with liquid water but react with water vapour. When the reaction happens, it results in the formation of a solid and a gas. The products of the reaction are Iron oxide and hydrogen. The equation of the reaction would be
Fe (s) + H₂O (g) → Fe₃O₄ (s) + H₂ (g)
Now, we need to balance the equation. On the right-hand side, we have 3 Fe, 4 O and 2 H. Similarly on the left-hand side there are 1 Fe, 1 O and 2 H.
To balance the equation, we add 3 to Fe, 4 to H₂0 and 4 to H₂.
As a result, the balanced chemical equation for the reaction would be
3 Fe (s) + 4H₂O (g) → Fe₃O₄ (s) + 4H₂ (g)
To know more about Balanced equation
https://brainly.com/question/12192253
#SPJ1
As the temperature of a given sample of a gas decreases at constant pressure, the volume of the gas
Answers
As the temperature of a given sample of an ideal gas decreases at constant pressure, the volume of the gas: increases in accordance with Charles's law.
Charles law states that when the pressure of an ideal gas is kept constant, the volume of the gas is indirectly proportional to the absolute temperature of the gas.
Mathematically, Charles law is given by the formula;
\(\frac{V}{T} =k\)
Where;
T is the temperature of an ideal gas.V is the volume of an ideal gas.According to Charles's law, when the volume of an ideal gas is decreased at a constant pressure, the temperature of the gas is increased and when the volume of a gas is increased at a constant pressure, the temperature of the gas is decreased due of the inverse relationship existing between them.
In conclusion, the temperature of a gas decreases the volume occupied by a gas provided pressure is kept constant in accordance with Charles's law.
Read more: https://brainly.com/question/22971882
Describe a method to separate the dyes in coloured inks. [4 marks]
A paper chromatogram from a mixture of two substances, A and B, was obtained using a solvent of propanone. Substance B was found to travel further up the paper than substance A.
What does this tell you about substances A and B. [1 mark]
Look at the boiling points of the three liquids in the table: Liquid Boiling point in °C water 100 ethanol 78 propanol 97 A mixture was made by stirring together equal volumes of these three miscible liquids. Evaluate the effectiveness of fractional distillation as a way of separating this mixture into the three pure liquids.
Answers
Chromatography is a method of separating out materials from a mixture.
Aim: To separate the dye present in ink by the process of evaporation.
Materials required: Beaker, watch glass, water, ink and stove.
Procedure: Take a beaker and fill it to half its volume with water. Keep 3, glass on the mouth of a beaker. Put few drops of ink on the watch glass. Heat the beaker and observe the watch glass.
Observations: We observe some fumes coming from the watch glass. Continue heating till you do not observe any further change on the watch glass. A small residue will be remained on the watch glass.
Inference: We know that ink is a mixture of a dye in water. The residue remained on the watch glass is the dye present in the ink.
Chromatography is a method of separating out materials from a mixture. Ink is a mixture of several dyes and therefore we can separate those colors from one another using chromatography.
Learn more about chromatography at
https://brainly.com/question/1558595
The mechanism for a set of reactions is:
a) Write a differential equation for the disappearance of A
b) Write a differntial equation for
c) Write a differential equation for the appearance of D.
d) if [A]o is the concentration of A at zero time, write an equation that gives [A] at any later time.
Answers
The mechanism for a set of reactions is given by
a) The differential equation is -d[A]/dt = k1[A]
b) Incomplete question
c) d[D]/dt = \(k_{2}\)[B][C] '
d)[A] = [A]₀ * e^(-\(k_{1}\)t)
How to write differential equations for various reactions?
a) To write a differential equation for the disappearance of A, we need to consider the rate at which A is disappearing. Let's denote the rate of disappearance as -d[A]/dt. The negative sign indicates that the concentration of A is decreasing over time. The differential equation will be:
-d[A]/dt = \(k_{1}\)[A]
b) Unfortunately, the question is incomplete, and the differential equation for which species is not specified. Please provide more information to answer this part.
c) To write a differential equation for the appearance of D, we need to consider the rate at which D is being produced. Let's denote the rate of appearance as d[D]/dt. The differential equation will be:
d[D]/dt = \(k_{2}\)[B][C]
d) To write an equation that gives the concentration of A at any later time, we need to solve the differential equation we found in part a):
-d[A]/dt = \(k_{1}\)[A]
Integrating both sides with respect to time, we get:
∫-d[A]/[A] = \(k_{1}\)∫dt
-ln[A] = \(k_{1}\)t + C
Now, we can solve for the constant C using the initial condition that [A] = [A]₀ at t = 0:
-ln[A]₀ = C
So, our equation for [A] at any later time t is:
-ln[A] = \(k_{1}\)t - ln[A]₀
Solving for [A], we have:
[A] = [A]₀ * e^(-\(k_{1}\)t)
To know more about Differential Equations:
https://brainly.com/question/31255580
#SPJ11
Wind and ocean currents do not move in straight lines; instead, they curve as they move across the planet. What is responsible for this pattern of movement? differences in water temperature differences in water salinity differences in water density the Coriolis effect
Answers
Answer:
the Coriolis effect
Explanation:
the Coriolis effect is a pattern of deflection responsible for this pattern of movement. since the earth rotates on it's axis, circulating air is deflected.
Answer: The Coriolis effect (make the other guy brainlyest he said it first)
Explanation:
Which of the following electrons configuration represents an element that is most likely to bond in a 1:1 ratio with calcium, Ca?
Answers
The electronic configuration that is most likely to bond with Calcium with the ratio of 1:1 is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s².
To make a bond of Calcium with 1:1 ratio the other element should satisfy the same valency of that of Calcium. By doing so the element Calcium can bond with the ratio of 1:1.
Then in such a case, the last shell of the element should be in a position to gain 2 electrons which would be lost by Calcium.
This would create a bond of 1:1 ratio. A best example for this would be Calcium Oxide (CaO). Here, the valency of Calcium is 2+ and valency of oxygen is 2- which forms a compound of ratio 1:1.
Therefore, the electronic configuration that is most likely to bond with Calcium in the ratio of 1:1 is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s².
To learn more about valence electrons, click below:
https://brainly.com/question/371590
#SPJ1
what is the difference between deep water and shallow water waves???
Answers
Answer:
The change from deep to shallow water waves occurs when the depth of the water , d , becomes less than one half of the wavelength of the wave, λ....A wave with a longer wavelength travels at higher speed . In contrast , shallow - water waves show no dispersion . Their speed is independent of their wavelength .
Hope this helps :)
In an ocean ecosystem,the difference between deep water and shallow water waves is that waves in water deep enough so that the bottom has no effect on them are termed deep-water waves, whereas waves most affected by the bottom are called shallow-water waves.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/1673533
#SPJ2