Answers
True
True
False
True
False
Hope this helps!
Related Questions
why was it so important that the electron was smaller than the atom?
Answers
Answer:
because electron is a negetively charge particle that orbits the outer part of atom and despite being small it is a strong proton.
Explanation:
Arthritis can be treated with which of these? Check all that apply.
anti-inflammatory medications
avoidance of sun exposure
joint replacement surgery
removal of the thyroid gland
regular exercise
Answers
Arthritis can be treated with anti-inflammatory medications, joint replacement surgery, and regular exercise.
What is Arthritis?Arthritis can be described as an inflammation of one or more joints, causing pain or stiffness that can worsen with age. Different kinds of arthritis exist, each with different causes of infections, including wear and tear, and underlying diseases.
Osteoarthritis occurs when the cartilage and tissues within the joint break down or have a change in structure but it does not occur because of wear and tear on the joints.
Common symptoms include swelling, pain, decreased range of motion, and stiffness. Anti-inflammatory Medications, physical therapy, or sometimes surgery helps decrease symptoms and improve quality of life.
Learn more about Arthritis, here:
https://brainly.com/question/14131904
#SPJ1
All are true about how does gravity aid erosion except?
Gravity causes rocks to fall and break apart.
Gravity causes rocks to get larger then breaks it apart.
Gravity causes other soil and sand to move down eroding rocks.
Gravity pulls rainwater down eroding rocks.
Answers
B) Gravity causes rocks to get larger then breaks it apart
hope this helps!
A FeCl 3 solution is 0.175 M. How many mL of a 0.175 M FeCl 3 solution are needed to make 650. mL of a solution that is 0.300 M in Cl - ion?
Answers
Based on the molar concentration, the volume of FeCl₃ required is 371 mL
What is the mole ratio of the reaction?The mole ratio of the reaction is determined from the equation of the reaction as follows:
Equation of reaction: FeCl₃ (aq) ----> Fe³⁺ (aq) + 3 Cl⁻ (aq)
From the equation of reaction, the mole ratio of FeCl₃ to Cl⁻ is 1 : 3
The moles of chloride ions in 650 mL of 0.300 M is calculated from the formula below:
Moles = molar concentration * volume in litersMoles of Cl⁻ ions = 0.300 * 650/100
Moles of Cl⁻ ions = 0.195
Moles of FeCl₃ required = 0.195/3
Moles of FeCl₃required = 0.065
volume of FeCl₃ required = 0.065 / 0.175
volume of FeCl₃ required = 0.371 L or 371 mL
Learn more about molar concentration at: https://brainly.com/question/14469428
#SPJ1
4-How does the concentration of ions in a strong acid differ from a weak acid?
Answers
Wich of these BEST shows a change from potential to kinetic energy?
Choices
A: A sandwich at lunch helps a students run a race.
B: Electricity making a bulb light up.
C: Transfer of heat trough electromagnetic waves.
D: A ball sitting at the top of the hill.
Answers
ammonia chemically reacts with oxygen gas to produce nitric oxide and water . what mass of nitric oxide is produced by the reaction of of oxygen gas?
Answers
They want to know the mass of nitric oxide that is produced by this reaction. The balanced chemical equation for this reaction is:
4NH₃(g) + 5O₂(g) → 4NO(g) + 6H₂O(g)
In this equation, 4 moles of NH₃ react with 5 moles of O₂ to produce 4 moles of NO and 6 moles of H₂O. To find the mass of NO produced, we need to use the molar mass of NO. Molar mass of NO = 30.01 g/molTo find the mass of NO produced, we need to use stoichiometry.
We know that 4 moles of NH₃ reacts with 5 moles of O₂ to produce 4 moles of NO. Therefore, if we know the number of moles of O₂ that reacted, we can use stoichiometry to find the number of moles of NO produced.We can use the ideal gas law to find the number of moles of O₂ that reacted.
n = PV/RT We can assume that the reaction took place at standard temperature and pressure (STP), which means:P = 1 atmV = 22.4 L (molar volume of an ideal gas at STP)T = 273.15 K (0 °C)R = 0.08206 L atm/(mol K)Using these values, we can find the number of moles of O₂ that reacted:n = PV/RT = (1 atm)(22.4 L)/(0.08206 L atm/(mol K) * 273.15 K) ≈ 1 mol
Therefore, 1 mole of O₂ reacted in the reaction. Using stoichiometry, we can find the number of moles of NO produced.4 moles NH₃ : 4 moles NO1 mole O₂ : 4/5 moles NO (from the balanced equation)1 mole O₂ was consumed, so the number of moles of NO produced is:1 mole O₂ * (4/5 moles NO/1 mole O₂) = 0.8 moles NO
Finally, we can find the mass of NO produced using the molar mass of NO:mass NO = number of moles * molar mass mass NO = 0.8 mol * 30.01 g/mol ≈ 24.0 g Therefore, approximately 24.0 grams of NO are produced by the reaction between ammonia and oxygen gas.
To learn more about nitric oxide here:
https://brainly.com/question/17092405
#SPJ11
Scientists may design an experiment with a control group, which is a set of organisms or sam-ples that do NOT receive the treatment (the independent variable) that is being tested. Scientists can then compare normal changes in organisms or samples with those that might have occurred because of the treatment. The idea of a "control group" is not the same as a "controlled variable." Suppose a scientist is doing an experiment to determine the effect of a cancer drug on mice with lymphoma
Answers
Answer:
See explanation
Explanation:
I believe that the aim of the scientist is to determine the effect of a cancer drug on mice with lymphoma. In this experiment, the mice with lymphoma are exposed to the drug. This is the treatment in the experiment. A control group of mice having lymphoma is not exposed to this treatment, this is the control group. This control group establishes a baseline for the study.
By comparing the outcome of the experimental and control groups, the effect of a cancer drug on mice with lymphoma can be determined.
____ equals the number of protons in the atom?
mass number
atomic number
electrical charge
Answers
What is S for silicon tetrachloride, SiCl4

Answers
S denotes the ''number of shared electron pairs by an atom'', Hence, Silicon shares its 4 electrons with 4 Cl-atoms. Thus the Value of S is 4 in SiCl₄ .It is also known as covalency
What is Covalency ?
The number of covalent bonds that a particular atom can make with other atoms in forming a molecule.
Covalent bond id formed by the sharing of electron between two atoms.
Hence, Silicon shares its 4 electrons with 4 Cl-atoms. Thus the Value of S is 4 in SiCl₄ . It is also known as covalency
Learn more about Covalency here ;
https://brainly.com/question/26152707
#SPJ1
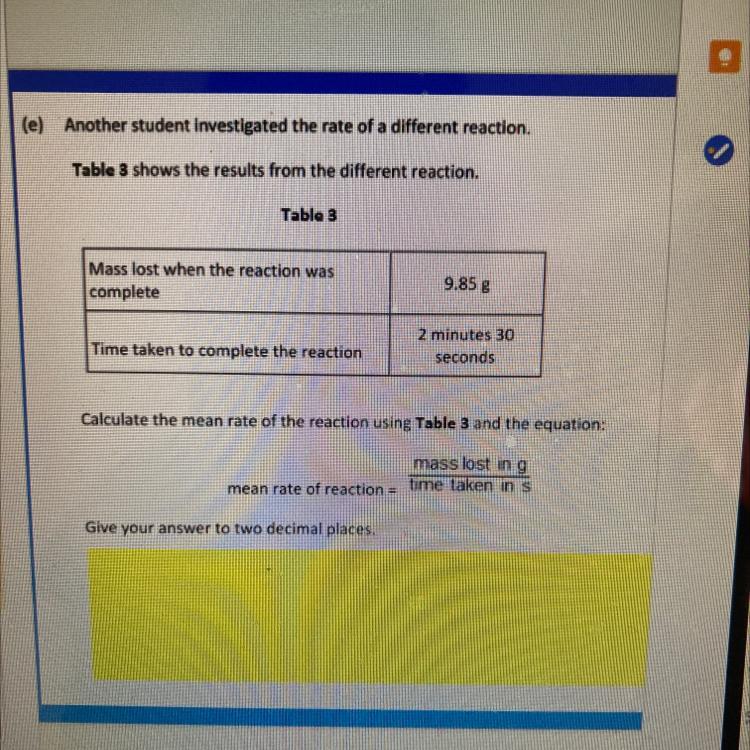
(e) Another student investigated the rate of a different reaction
Table 3 shows the results from the different reaction
Headings that you a
will appear here.
Table 3
Mass lost when the reaction was
complete
9.85 g
Time taken to complete the reaction
2 minutes 30
seconds
Calculate the mean rate of the reaction using Table 3 and the equation:
mass lost in g
mean rate of reaction time taken in s
Give your answer to two decimal places.
Answers
Answer:
0.07 g/s.
Explanation:
From the question given above, the following data were obtained:
Mass lost = 9.85 g
Time taken = 2 min 30 s
Mean rate =?
Next, we shall convert 2 min 30 s to seconds (s). This can be obtained as follow:
1 min = 60 s
Thus,
2 min = 2 × 60 = 120 s
Therefore,
2 min 30 s = 120 s + 30 s = 150 s
Finally, we shall determine the mean rate of the reaction. This can be obtained as illustrated below:
Mass lost = 9.85 g
Time taken = 150 s
Mean rate =?
Mean rate = mass lost / time taken
Mean rate = 9.85 / 150
Mean rate = 0.07 g/s
Therefore, the mean rate of the reaction is 0.07 g/s
How many atoms are in a 0.50 g sample of helium
Answers
how many unique sets of 4 quantum numbers are there to represent the electrons in the 4f subshell? remember that the pauli exclusion principle states that each electron must have its own unique set of 4 quantum numbers.
Answers
The number of unique sets of 4 quantum numbers to represent the electrons in the 4f subshell is 70.
The four quantum numbers that make up an electron's set are the:
(i) principal quantum number (n)
(ii) angular momentum quantum number (l)
(iii) magnetic quantum number (m_l)
(iv) spin quantum number (m_s).
Each of these electrons has a limited range of the above numbers in their respective shell.
The principal quantum number for all the electrons in the 4f subshell is 4.
The angular momentum quantum number has a value of 3 corresponding to the f subshell.
The magnetic quantum number has a range of -3 through +3 for the electrons in the f subshell.
The spin quantum number has a range of -1/2 or +1/2.
Even if the principal quantum number and angular momentum quantum number are the same for all the electrons, the other two factors contribute to each electron having a unique set of quantum numbers.
Therefore, when these four quantum numbers are combined, they make up 70 unique sets of 4 quantum numbers that can be used to represent the electrons in the 4f subshell, in accordance with the Pauli Exclusion Principle.
To know more about quantum numbers, refer here:
https://brainly.com/question/16977590#
#SPJ11
What are some ways each person could reduce air pollution and energy consumption? a. adjust the temperature settings - hotter in winter and cooler in summer b. adjust the temperature settings - cooler in winter and hotter in summer c. cut down trees near the house d. replace plumbing fixtures that leak Please select the best answer from the choices provided A B C D
Answers
Answer:
b.
adjust the temperature settings - cooler in winter and hotter in summer
Explanation: I took the quiz
Answer:
B. adjust the temperature settings- cooler in winter and hotter in summer
Explanation:
edge 2020
What are the various methods to make fabric? Explain
Answers
Explanation: hope this help
Mark as brainlist
how many grams of sodium chloride (mw = 58.4) are needed to make .5 l of a 0.15 m solution?
Answers
The grams of sodium chloride are needed to make 0.5 l of a 0.15 M solution is 4.38 g.
The molarity of the sodium chloride solution = 0.15 M
The volume of the sodium chloride solution = 0.5 L
The number of the moles of the sodium chloride = molarity × volume
The number of the moles of the sodium chloride = 0.15 × 0.5
The number of the moles of the sodium chloride = 0.075 mol
The molar mass of the sodium chloride = 58.4 g/mol
The mass of the sodium chloride = moles × molar mass
The mass of the sodium chloride = 0.075 × 58.4
The mass of the sodium chloride = 4.38 g
To learn more about moles here
https://brainly.com/question/26416088
#SPJ4
show how the nucleophile for the wittig reaction was generated from the phosphonium salt. draw the reaction mechanism. use curved-arrow notation. include resonance structures (if any)
Answers
The phosphonium salt was used to produce the nucleophile for the reaction, which is Ph3P = CH2. The following reaction produces the nucleophile: PPh3 + Ch3 -I Ph3p+ - ch3.
Where does phosphonium come from?By ring-opening nucleophilically adding tertiary phosphines to cyclic compounds, it is possible to create quaternary phosphonium salts. A betaine (57), which is the first product, is protonated in the presence of acid to produce the phosphonium salt (58).
What exactly is phosphonium ion?Although the phosphonium ion PH4+ is structurally similar to the ammonium ion NH4+, PH3 has a significantly lower affinity for protons than NH3, making the interaction of PH3 with acids essential to produce phosphonium salts. from: A Dictionary of Chemistry, entry on phosphonium ion Chemistry is a topic in science and technology.
To know more about Phosphonium visit:
https://brainly.com/question/29098429
#SPJ4
Por favor, necesito ayuda, es urgente!!!

Answers
Answer:
what type of language this is ??
determine the ph of a 0.741 m lioh solution at 25°c.
Answers
The pH of the 0.741 M LiOH solution at 25°C is approximately 13.87.
To determine the pH of a 0.741 M LiOH solution at 25°C, we need to calculate the concentration of hydroxide ions (OH-) in the solution and then use that value to find the pOH. Finally, we can calculate the pH using the equation pH = 14 - pOH.
LiOH is a strong base that dissociates completely in water, so the concentration of hydroxide ions is equal to the concentration of LiOH. In this case, the concentration is given as 0.741 M.
Next, we can calculate the pOH using the equation pOH = -log[OH-]. Since the concentration of hydroxide ions is 0.741 M, the pOH is -log(0.741) = 0.13.
Finally, we can calculate the pH using the equation pH = 14 - pOH. Substituting the value of pOH, we get pH = 14 - 0.13 = 13.87.
Therefore, the pH of the 0.741 M LiOH solution at 25°C is approximately 13.87.
Know more about pH here:
https://brainly.com/question/2288405
#SPJ11
At 500 K, 1 mol of ONCl (g) is introduced in a 1 litre container. At equilibrium, 9
%
of the ONCl is dissociated: 2ONCl (g) ⇌
2NO (g) + Cl2 (g).
What is the equilibrium constant K?
Answers
The reaction is 2NOCl (g) ⇌2NO (g) + Cl₂ (g). The expression for the equilibrium constant will be 4.337 × 10⁻⁴ M
What is the equilibrium constant?The forward reaction velocity constant and the backward reaction velocity constant are multiplied to produce the equilibrium constant. According to some sources, it is "the ratio of product to the molar concentration of product to product of the molar concentration of reactants." The difference between the equilibrium product and reactant concentrations is the equilibrium constant of a reaction. The concentration terms are elevated to suitable powers that are equivalent to stoichiometric coefficients.
Given, the reaction is 2NOCl (g) ⇌2NO (g) + Cl₂ (g).
K = \(\frac{[NOCl^2]}{[NO]^2 [Cl2]}\)
Δ \(n_{g}\) = (2+1) -2 = 1
Temperature T, 500 K
\(K_{p}\) value = 1.8 × 10⁻² bar⁻¹
Now
\(K_{p} = K_{c} (RT)^{-ng}\)
= 1.8 × 10⁻² × (0.083 × 500)⁻¹M
= 4.337 × 10⁻⁴ M
To learn more about equilibrium constant, visit:
https://brainly.com/question/3042203
#SPJ1
Whose discovery led to the discovery of the proton?
A. Dalton
B. Rutherford
C. Aristotle
D. Bohr
Answers
Balance the equation K2SO4 + HNO3 ->KNO3 + H2SO4 what's the final balanced equation
Answers
Answer:
2KNO3 + H2SO4 → K2SO4 + 2HNO3
Explanation:
Hope this helps
describe the stabilisation and destabilisation of octahedral complexes
Answers
The stabilization and destabilization of octahedral complexes refer to the changes in the energy levels of d-orbitals in transition metal complexes, which affects their properties and reactivity.
In octahedral complexes, the d-orbitals of the central metal ion split into two sets of energy levels due to the presence of ligands. This is known as crystal field splitting. The energy gap between these sets is determined by the strength of the ligand field, which is related to the nature of the ligands and the geometry of the complex.
Stabilization occurs when the ligand field is strong, causing a large energy gap between the two sets of orbitals (t2g and eg). This leads to lower energy and more stable complexes. Examples of strong ligands that cause stabilization include CN-, CO, and NO2-.
Destabilization, on the other hand, occurs when the ligand field is weak, causing a smaller energy gap between the sets of orbitals. This leads to higher energy and less stable complexes. Examples of weak ligands that cause destabilization include I-, Br-, and Cl-.
In summary, the stabilization and destabilization of octahedral complexes are determined by the ligand field strength and the resulting energy gap between the d-orbitals, affecting the properties and reactivity of the complexes.
Learn more about splitting here:
https://brainly.com/question/12880656
#SPJ11
Answer is B
A metal conducts heat in a similar way to electricity so, as you touch it, it draws heat from your body and makes your hand feel cold.
Plastics don't have to conduct heat so they don't draw heat from your body as efficiently, that's why a piece of plastic feels
than a piece of metal even if it's at the same temperature.
A)
long-chain molecules; warmer
B)
free electrons, warmer
valence electrons; colder
D)
a crystalline lattice; colder
Answers
B)
free electrons, warmer
valence electrons; colder
There becomes a relationship between all of the species in a community. This
relationship is called a
Answers
one mole of an ideal gas expands reversibly and isothermally at temperature t until its volume is doubled. the change of entropy of this gas for this process is:
Answers
The change of entropy for one mole of an ideal gas expanding reversibly and isothermally until its volume is doubled is equal to the gas constant (R) multiplied by the natural logarithm of 2.
]The change of entropy (ΔS) for one mole of an ideal gas that expands reversibly and isothermally at temperature T, with its volume doubling, is given by the formula: ΔS = nR ln(V2/V1). In this case, n = 1 mole, V2 = 2V1, and T remains constant.
To calculate the change of entropy for this specific process, we will use the given conditions and the formula mentioned above. As the process is isothermal, the temperature (T) remains constant throughout the process. Moreover, the process is reversible, which means that the gas is in equilibrium with its surroundings at every point of expansion. The volume of the gas doubles, so the final volume (V2) is twice the initial volume (V1).
Using the formula ΔS = nR ln(V2/V1), we can substitute the given values:
ΔS = (1 mole) * R * ln(2V1/V1).
Since V2 = 2V1, the formula simplifies to:
ΔS = R * ln(2).
Thus, the change of entropy for one mole of an ideal gas expanding reversibly and isothermally until its volume is doubled is equal to the gas constant (R) multiplied by the natural logarithm of 2.
Know more about Entropy here:
https://brainly.com/question/13135498
#SPJ11
The highest mountain in North America is Mt. McKinley at 3.848 miles high. How many kilometers is that?
Answers
John Dalton's original atomic theory contain the following key ideas. Which part(s) of these ideas were was incorrect?
A. elements are made of tiny indivisible particles called atoms
B. Atoms are unchanged in chemical reaction
C. Atoms can join together in whole number ratios to form compounds
D. The atoms of each element are unique
Answers
John Dalton's original atomic theory proposed that elements are made up of tiny indivisible particles called atoms, and that these atoms are unchanged in chemical reactions. It also stated that atoms can combine in whole number ratios to form compounds, and that the atoms of each element are unique.
However, with the advancements in science, we now know that one part of Dalton's theory was incorrect. Atoms are not indivisible, as they can be split into smaller subatomic particles, such as protons, neutrons, and electrons. Furthermore, atoms can also be changed in chemical reactions, as they can lose or gain electrons, or undergo nuclear reactions.
Therefore, while John Dalton's theory provided a solid foundation for the understanding of atoms and elements, it was incomplete and required further refinement through scientific exploration and discovery.
To learn more about atomic visit;
https://brainly.com/question/1566330
#SPJ11
the molar solubility of c a ( o h ) 2 was experimentally determined to be 0.021 m. based on this value, what is the k s p of c a ( o h ) 2 ?
Answers
The Ksp of Ca(OH)₂ is approximately 3.71 × 10⁻⁵.
To find the Ksp of Ca(OH)₂ based on the molar solubility of 0.021 M, you can follow these steps:
1. Write the balanced dissociation equation for Ca(OH)₂ :
Ca(OH)₂ (s) ⇌ Ca²⁺ (aq) + 2OH⁻ (aq)
2. Since the molar solubility of Ca(OH)₂ is 0.021 M, that means:
[Ca²⁺] = 0.021 M
[OH⁻] = 2 × 0.021 M = 0.042 M (because there are 2 moles of OH⁻ for every 1 mole of Ca²⁺)
3. Write the Ksp expression for the dissociation of Ca(OH)2:
Ksp = [Ca²⁺] × [OH⁻]²
4. Substitute the molar solubility values into the Ksp expression:
Ksp = (0.021) × (0.042)²
5. Calculate the Ksp value:
Ksp = (0.021) × (0.001764) = 3.7064 × 10⁻⁵
So, the Ksp of Ca(OH)₂ is approximately 3.71 × 10⁻⁵.
Learn more about molar solubility at https://brainly.com/question/28202068
#SPJ11
wont more BRAINLYS just answer the question right

Answers
Answer:
1. A.
2. D.
3. E.
4. B
5. C.
Explanation:
1. The water cycle uses radiant energy from the sun to function
2. Crystals form by crystalization, hence the name.
3. Condensation is when water vapor changes to a liquid.
4. The water cycle is the movement of water on earth on and below it's crust.
5. Transperation is how plants release water into the air. Also how humans sweat.
Have a most wonderous day!
