Answers
Answer:
+2
Explanation:
All the alkaline earth metals have 2 valence electrons in their valence shell. Therefore, they lose 2 electrons to form cations with a +2 charge. For example, Mg²⁺.
Hope that helps.
Related Questions
For each of these pairs of half-reactions, write the balanced equation for the overall cell reaction and calculate the standard cell potential. Express the reaction using cell notation. You may wish to refer to Chapter 20 to review writing and balancing redox equations.
1.
Pt2+(aq)+2e-Pt(s)
Sn2+(aq)+2e-Sn(s)
2.
Co2+(aq)+2e-Co(s)
Cr3+(aq)+3e-Cr (s)
3.
Hg2+(aq)+2e-Hg (I)
Cr2+(aq)+2e-Cr (s)
please help out
Answers
1. For the pair of half-reactions:
Pt2+(aq) + 2e- → Pt(s) ... (1)
Sn2+(aq) + 2e- → Sn(s) ... (2)
To obtain the balanced equation for the overall cell reaction, we need to multiply the half-reactions by appropriate coefficients to ensure that the number of electrons transferred is equal. In this case, we can multiply equation (1) by 2 and equation (2) by 1:
2(Pt2+(aq) + 2e-) → 2(Pt(s))
Sn2+(aq) + 2e- → Sn(s)
Combining the equations, we have:
2Pt2+(aq) + Sn2+(aq) → 2Pt(s) + Sn(s)
The cell notation for this reaction is:
Pt2+(aq) | Pt(s) || Sn2+(aq) | Sn(s)
To calculate the standard cell potential (E°), we need to know the standard reduction potentials for Pt2+/Pt(s) and Sn2+/Sn(s) half-reactions. Referring to standard reduction potential tables, we find:
E°(Pt2+/Pt(s)) = +1.20 V
E°(Sn2+/Sn(s)) = -0.14 V
The overall cell potential (E°cell) is the difference between the reduction potentials:
E°cell = E°(cathode) - E°(anode) = 0.00 V - (-0.14 V) = +0.14 V
Therefore, the standard cell potential for this reaction is +0.14 V.
2. For the pair of half-reactions:
Co2+(aq) + 2e- → Co(s) ... (3)
Cr3+(aq) + 3e- → Cr(s) ... (4)
To balance the number of electrons transferred, equation (4) can be multiplied by 2:
2(Co2+(aq) + 2e-) → 2(Co(s))
Cr3+(aq) + 3e- → Cr(s)
Combining the equations, we have:
2Co2+(aq) + Cr3+(aq) → 2Co(s) + Cr(s)
The cell notation for this reaction is:
Co2+(aq) | Co(s) || Cr3+(aq) | Cr(s)
To calculate the standard cell potential (E°), we refer to the standard reduction potentials:
E°(Co2+/Co(s)) = -0.28 V
E°(Cr3+/Cr(s)) = -0.74 V
The overall cell potential (E°cell) is the difference between the reduction potentials:
E°cell = E°(cathode) - E°(anode) = -0.74 V - (-0.28 V) = -0.46 V
Therefore, the standard cell potential for this reaction is -0.46 V.
3. For the pair of half-reactions:
Hg2+(aq) + 2e- → Hg (l) ... (5)
Cr2+(aq) + 2e- → Cr(s) ... (6)
The equation for the overall cell reaction can be obtained by multiplying equation (6) by 2:
2(Hg2+(aq) + 2e-) → 2(Hg (l))
Cr2+(aq) + 2e- → Cr(s)
Combining the equations, we have:
2Hg2+(aq) + Cr2+(aq) → 2Hg (l) + Cr(s)
For more such questions on balanced equation.
https://brainly.com/question/11904811
#SPJ8
An empty balloon sits 10 meters away from a golf ball. Jamie wants to increase the
gravitational force between the two objects by filling the balloon with a substance. Which
of the following substances will most likely increase the gravitational force between the
balloon and the golf ball?
An empty balloon sits 10 meters away from a golf ball. Jamie wants to increase the
gravitational force between the two objects by filling the balloon with a substance. Which
of the following substances will most likely increase the gravitational force between the
balloon and the golf ball?
water
cotton
air
lead pieces
Answers
To increase the gravitational force between the balloon and the golf ball, It should be filled with lead pieces. Option D
What should be done?A substance's density, which measures its mass in relation to its volume, determines how much gravitational force it produces.
Lead bits are one of the suggested materials, and they are the one that would most likely boost the gravitational force. The density of lead is much higher than that of the other listed materials.
The high density of lead will result in an increase in the gravitational pull between the balloon and the golf ball if Jamie fills the balloon with lead bits.
Learn more about density:https://brainly.com/question/29775886
#SPJ1
If 5 g of sodium chloride saturates 12.5 g of water at 10 °C, what mass of sodium chloride would saturate 50 g of water at constant temperature?
Answers
Explanation:
since 5g saturates 12.5g of water at 10°c
so......x would saturate 50g of water at 0°c
then you can cross multiply
How many mg of water are present in a 500. mg sample of 2.9 m/m% solution?
Report your answer to 3 significant figures and without units.
Answers
The answer is 14.5 mg.
To solve the problem, we need to first understand what a 2.9 m/m% solution means. "m/m%" stands for mass per mass percentage, which indicates the mass of the solute (in this case, water) divided by the total mass of the solution (water + solvent, which is not specified). Therefore, a 2.9 m/m% solution means that for every 100 grams of the solution, 2.9 grams are water.
To find the amount of water present in a 500 mg sample of this solution, we can set up a proportion:
2.9 g water/100 g solution = x g water/500 mg solution
Simplifying this proportion by converting units to milligrams, we get:
2.9 mg water/100 mg solution = x mg water/500 mg solution
Cross-multiplying and solving for x, we get:
x = 14.5 mg
Therefore, there are 14.5 mg of water present in a 500 mg sample of 2.9 m/m% solution.
how does the density of an object help scientist understand the natural world
Answers
Densities are mostly used to identify substances and to characterize and estimate the composition of many kinds of mixtures.
In which of the following, are all the elements non-metals?
A. Na, Mg, O, N
B. C, Si, Ge, As
C. Fe, Ni, Cr, O
D. He, Ne, Ar, Kr
E. Ca, Ba, Sr, S
Answers
Answer:
The answer is D
Explanation:
Non metals are:
Hydrogen (H)
Sulphur (S)
Phosphorus (P)
Carbon (C)
Fluorine (F)
Oxygen (O)
Nitrogen (N)
Chlorine (Cl)
Bromine (Br)
Helium (He)
Argon (Ar)
Iodine (I)
Neon (Ne)
Krypton (Kr)
Radon (Rn)
Selenium (Se)
Xenon (Xe)
what is the chemistry
Answers
Sound waves can travel across outer space true or false
Answers
Answer:
False
Explanation:
sounds waves can't travel so far
Explanation: Scientist thinks that there is actually sound in space it’s just that the human ear can’t hear it.
State the law and solve the problem.
A gas has a volume of 550 mL at a temperature of 5C. What volume will the gas occupy at 30C,
assuming constant pressure?
Remember significant figures and units.
6 POINTS
Answers
The law involved is Charles law and the volume the gas will occupy at 30°C is 599.46mL. Details about Charles law can be found below.
How to calculate volume?Charles law gives the relationship between the volume of a gas and its temperature. The volume of a gas can be calculated using the following equation:
V1/T1 = V2/T2
Where;
V1 = initial volume = 550mLT1 = initial temperature = 5°CV2 = final volume = ?T2 = final temperature = 30°C550/278 = V2/303
303 × 550 = 278V2
166,650 = 278V2
V2 = 599.46mL
Therefore, the law involved is Charles law and the volume the gas will occupy at 30°C is 599.46mL.
Learn more about volume at: https://brainly.com/question/1578538
#SPJ1
2.How might the structure of molecules help scientists determine how they interact with other molecules?
Answers
According to the molecular geometry, with the help of structure of molecules which provide information on site of attachment with other molecules, one can determine their mode of reaction.
What is molecular geometry?Molecular geometry can be defined as a three -dimensional arrangement of atoms which constitute the molecule.It includes parameters like bond length,bond angle and torsional angles.
It influences many properties of molecules like reactivity,polarity color,magnetism .The molecular geometry can be determined by various spectroscopic methods and diffraction methods , some of which are infrared,microwave and Raman spectroscopy.
Learn more about molecular geometry,here:
https://brainly.com/question/7558603
#SPJ1
Answer: The shape of a molecule helps to determine its properties, which affect how a molecule interacts with other molecules, such as polarity and bonding.
In the reaction below, 1.000 × 103 g LiOH is combined with 8.80 × 102 g CO2. The reaction produces 3.25 × 102 g H2O in an experiment. CO2 + 2LiOH → Li2CO3 + H2O Type in the answer using 3 significant figures. %
Answers
The mass of water produced when 1.000 × 10³ g of LiOH is combined with 8.80 × 10² g \(CO_{2}\) is 3.25 × 10² g\(H_{2}O\).
What mass of water is produced when lithium hydroxide and carbon dioxide react?The reaction between lithium hydroxide and carbon dioxide to produce water and lithium carbonate is a neutralization reaction.
The equation of the reaction is given below:
\(CO_{2} + 2\:LiOH \rightarrow Li_{2}CO_{3} + H_{2}O\)When 1.000 × 10³ g of LiOH is combined with 8.80 × 102 g \(CO_{2}\). The reaction produces 3.25 × 10² g\(H_{2}O\).
Learn more about neutralization reaction at: https://brainly.com/question/21582313
#SPJ1
Answer:CO2
Explanation: Hope this helps.
write the products that form for the following reaction Al + Ca(NO3)2
Answers
The following balanced chemical equation may be used to describe the interaction between aluminum (Al) and calcium nitrate (Ca(NO₃)₂):
2 Al + 3 Ca(NO₃)₂ → 2 Al(NO₃)3 + 3 Ca
Reactants are the chemicals that begin a chemical reaction, while products are the compounds that are created as a result of the reaction.
The substances that initiate a chemical reaction. Products are the substances that are created during the reaction. Compounds or elements can act as reactants and products.
Aluminium and calcium nitrate interact in this reaction to form aluminium nitrate (Al(NO₃)₃) and calcium (Ca), which are the end products.
Learn more about chemical equation, here:
https://brainly.com/question/28972826
#SPJ1
How many grams are there in 1.4 x 1024 molecules of NH3?
Answers
2.32 atoms are there in 1.4 x 10²⁴. The smallest unit of matter with properties like chemical elements is the atom.
The atom represents the smallest portion of material that may be split without producing particles with an electrical charge. Essentially a result, an atom acts as the basic building block of chemistry. An atom is mostly made of space. The remaining material consists of a negatively charged cloud of electrons revolving about a positively charged nucleus composed of protons plus neutrons.
Number of atoms = 1.4 x 10²⁴/ 6.022×10²³
= 2.32 atoms
To know more about atom, here:
https://brainly.com/question/1566330
#SPJ1
Name the structure CH3coch2c(br)2ch2ch3
Answers
The name of the compound from the structure that we can see in the question is;
4,4-dibromohex-2one
Summary of how you name an organic compoundThe International Union of Pure and Applied Chemistry (IUPAC) established a systematic set of guidelines for naming organic compounds.
Find the compound's longest continuous chain of carbon atoms. The compound's name is derived from this chain, which also acts as the compound's parent chain.
Assign a number to each carbon atom in the parent chain to give each carbon atom in the compound a special identification. The end that is closest to the functional group or substitutes is where the numbering begins.
Learn more about organic compound:https://brainly.com/question/13508986
#SPJ1
Which property of a rock determines its color?
its grain size
its method of formation
its mineral composition
its coarse-grain content
Answers
Answer:
its mineral composition
Explanation:
The atomic bonds within a mineral generally determine which wavelengths of light will be absorbed and which will be reflected. Those wavelengths that are reflected back to our eyes determine the color of the mineral. Some minerals have free electrons that will absorb certain wavelengths of light.
Mineral composition is the property of a rock that determines it's color.
What is a rock?A rock is a naturally occurring solid that comprises of different minerals or substances which fuses together or solidified together to form a very big solid mass.
Therefore, Mineral composition is the property of a rock that determines it's color.
Learn more about rock below.
https://brainly.com/question/15663649
#SPJ5
Calculate the amount of copper in moles in a 27.5g pure copper sheet
Answers
The amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To calculate the amount of copper in moles in a pure copper sheet, we need to use the molar mass of copper and the given mass of the sheet.
The molar mass of copper (Cu) is approximately 63.55 g/mol. This value represents the mass of one mole of copper atoms.
Given that the mass of the pure copper sheet is 27.5 g, we can calculate the number of moles using the following formula:
moles = mass / molar mass
Substituting the values:
moles = 27.5 g / 63.55 g/mol
moles ≈ 0.433 mol
Therefore, the amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To arrive at this result, we divided the given mass of the sheet (27.5 g) by the molar mass of copper (63.55 g/mol). This calculation allows us to convert the mass of the sheet into the corresponding number of moles of copper.
The result tells us that the 27.5 g pure copper sheet contains approximately 0.433 moles of copper atoms. This conversion to moles is useful in various chemical calculations and allows for easier comparison and analysis of quantities on a molecular scale.
for more such question on copper visit
https://brainly.com/question/29176517
#SPJ8
How many elements are in the compound C2H8O
Answers
Answer:
I think 2
Explanation:
The density of titanium is 4.50 g/cm3 . What is the edge length (in cm ) of a titanium cube that contains 2.23×1024 titanium atoms? Express your answer to three significant figures.
Answers
Answer:
3.37 cm
Step-by-step:
The edge length of the titanium cube can be calculated using the formula:
Edge length = (Volume of cube)^(1/3)
And the volume of the cube can be calculated as follows:
1. Calculate the mass of the titanium atoms in the cube.
The mass of one titanium atom can be calculated by dividing the molar mass of titanium by Avogadro's number:
Mass of one titanium atom = Molar mass of titanium / Avogadro's number
= 47.867 g/mol / (6.022 × 10^23 atoms/mol)
= 7.943 × 10^-23 g/atom
The total mass of the titanium atoms in the cube is then:
Total mass of titanium atoms = (2.23 × 10^24 atoms) × (7.943 × 10^-23 g/atom)
= 1.773 × 10^2 g
2. Calculate the volume of the titanium cube.
The volume of the cube can be calculated by dividing the total mass of the titanium atoms by the density of titanium:
Volume of cube = Total mass of titanium atoms / Density of titanium
= 1.773 × 10^2 g / 4.50 g/cm^3
= 39.4 cm^3
3. Calculate the edge length of the titanium cube.
Finally, the edge length of the cube can be calculated as:
Edge length = (Volume of cube)^(1/3)
= (39.4 cm^3)^(1/3)
= 3.37 cm
So the edge length of the titanium cube is 3.37 cm, rounded to three significant figures.
Hope this helps!
A 0.032 mole sample of gas is contained in a 1 L container at 23 degrees Celsius. What is the pressure of the gas?
Answers
Ideal gas law is valid only for ideal gas not for vanderwaal gas. The pressure of 0.032 mole sample of gas is contained in a 1 L container at 23 degrees Celsius is 0.77atm.
What is ideal gas equation?
Ideal gas equation is the mathematical expression that relates pressure volume and temperature.
Mathematically,
PV=nRT
where,
P = pressure=?
V= volume=1 L
n =number of moles= 0.032 mol
T =temperature = 23°C=296K
R = Gas constant = 0.0821 L.atm/K.mol
P × 1 L = 0.032mole× 0.0821 L.atm/K.mol × 296K
P =0.77atm
Therefore, the pressure of 0.032 mole sample of gas is contained in a 1 L container at 23 degrees Celsius is 0.77atm.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ1
What's the name of this compound?

Answers
Answer:
2-methyl propane
Explanation:
that's the name
Part C
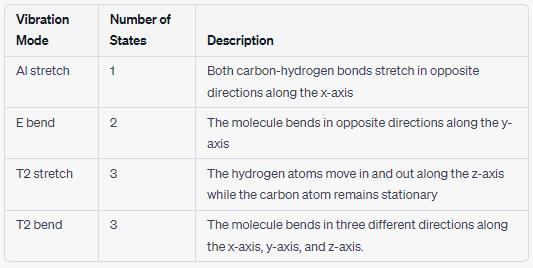
Close the simulation window for carbon dioxide and return to the Cool Molecules Explore page.
Next, click C and H in the periodic table and repeat the process for methane (CH₂). In the table below, record the names of its vibrational modes
and describe the vibration of the molecule in each mode. Also record the number of unique possible states for each mode.
Vibration Mode
Al stretch
E bend
T2 stretch
T2 bend
Number of States in the:
Mode
Description:
Answers
The vibrational modes and unique possible states for each mode are detailed in the table below.
What is the vibrational mode of a molecule?A vibrational mode of a molecule refers to a specific way in which the atoms inside a molecule can move relative to each other. Molecules are made up of atoms that are connected to each other by chemical bonds, and these bonds act like springs that can vibrate.
When a molecule absorbs energy, it can cause the bonds to stretch, bend, or twist in specific ways, creating different vibrational modes. The vibrational modes of a molecule can provide important information about its structure and chemical properties.
Find out more on vibrational modes here: https://brainly.com/question/30936396
#SPJ1
Nuclear changes lab
How does energy change in these reactions? Is energy needed to start the reactions or is energy given off in the reactions? For each type of reaction, approximately how much energy is released?
How do these energy changes compare in scale to other types of reactions, such as chemical reactions?
Answers
Energy is released during nuclear reactions. To calculate energy changes, use the attached image below. Nuclear reactions produce far more energy than other types of reactions, such as chemical reactions.
What is nuclear reaction ?The term nuclear reaction is defined as a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides.
Normally, chemical reactions take place outside the nucleus. Nuclear reactions occur only within the nucleus. When chemical reactions occur, elements retain their identity, as do the nuclei of atoms.
Thus, in nuclear reactions, the nuclei of atoms undergo complete transformations, resulting in the formation of new elements.
The nuclear reactions are as follows:
Alpha decay - PO₈₄²°⁶⇒ ₈₂Pb²°⁶ + He⁴₂
Beta⁻ decay - Na²²₁₁ ⇒ Na ²²₁₀ + e°₋₁
Beta⁺ decay - Na²⁴₁₁ ⇒ Na₁₀²⁴ + e°₋₁+β
Gamma - CO⁶°₂₇ ⇒ Ni⁶⁰₂₆ + e°₋₁ +γ
Fission - U²³⁵₉₂ + n₀¹ ⇒ Ba¹⁴⁴₅₆ + Kr⁸⁹₃₆ +3n₀¹
Fusion- H₁² +H³₁ ⇒ He⁴₂ + n¹₀ +17.59MeV
To learn more about the nuclear reaction, follow the link;
https://brainly.com/question/16526663
#SPJ9
Which of the following explains why a high mountain peak near the equator could have snow on it all year long?
Group of answer choices
water
wind
altitude
latitude (It's actually science btw but i didn't find it )
Answers
A high mountain peak near the equator could have snow on it all year long because of the altitude. That is option C.
What is equator?Equator is defined as the imaginary line of latitude that divides the earth into Northern and Southern hemispheres.
There are various regions of the earth that are located near the equator which experiences the equatorial climate throughout the year.
As the the high mountain peak near the equator, they could have snow on it all year long because of increased altitude.
This shows that air around this region becomes thinner and lacks the ability to absorb and retain heat leading to the presence of snow on the mountain top all year long.
Learn more about altitude here;
https://brainly.com/question/27816517
#SPJ1
Why atoms in our body don’t
eject electrons?
Answers
Answer:
When there are too many protons, some of the outer protons are loosely bound and more free to react with the electron. But most atoms do not have too many protons, so there is nothing for the electron to interact with. As a result, each electron in a stable atom remains in its spread-out wavefunction shape.
In an atom, there is a nucleus made up of neutral charged, neutrons, and positively charged protons. The reason why electrons which are negatively charged, don't fly off is due to its stronger attraction to the protons. ... This, however, still allows the electrons to move around the nucleus of an atom.
the product of a reaction between 1-propene and hydrobromic acid will result in which of the following?
Answers
2-Bromopropane is created when hydrobromic acid and propene undergo an addition process. The identical reaction results in 1 bromopropane when benzoyl peroxide is present.
Explain the Markovnikov rule using the reaction between propene and HBr.While the halide group attaches to the carbon atom with the most alkyl substituents when a protic acid (HX) is introduced to an asymmetric alkene, the acidic hydrogen adheres to the carbon with the most hydrogen substituents.
What is accurate for the alkene-HBr reaction in the presence of peroxide?The free-radical mechanism governs the addition reaction of HBr to an asymmetrical alkene in the presence of peroxide. Here, bromine radical is formed which reacts with an alkene and produces an anti-markovnikov product.
To learn more about hydrobromic acid visit:
brainly.com/question/15231576
#SPJ4
Which statement about subtatomic particles are true
Answers
The statement about sub-atomic particles that is true is:
Electrons are the subatomic particles with the smallest mass; option BWhat are sub-atomic particles?Sub-atomic particles refer to the smaller particles that are the constituents of atoms of elements.
There are three sub-atomic particles within a given atom,
The three sub-atomic particles are given below:
electrons -electrons are the negatively charged particles found outside the nucleus of an atom in electron shells or orbitals around the atom.protons - these are positively charged particles found inside the nucleus of an atom. The number of protons in an atom gives the atomic number of that atom.neutrons - these are neutral atoms found in the nucleus of atoms. The sum of the neutrons and protons in the nucleus of an atom gives the mass number of an atom. This is because the mass of an at is centered inside the nucleus since the proton and neutron have equal mass but the electron has negligible mass.Considering the given statements about the sub-atomic particles, the only true statement is that which describes the mass of the electron as being the smallest of the three sub-atomic particles.
Learn more about sub-atomic particles at: https://brainly.com/question/16847839
#SPJ1
Complete question:
Which statement about subatomic particles is true?
Protons are the only subatomic particles to have a charge.
Electrons are the subatomic particles with the smallest mass.
Neutrons orbit the nucleus of the atom.
Subatomic particles all have the same mass
How can you avoid the formation of a supersaturated solution?
Answers
How to make super saturated solution?
An aqueous solution can be rendered supersaturated by first dissolving the solute in water at an elevated temperature using enough to give a concentration just under its solubility at that temperature. After the last of the solute crystals have dissolved the solution is cooled.
Answer:
By adding water i guess
The contents of two vessels are combined as shown. The balanced chemical equation for the reaction that occurs is also shown. Which of the following represents the constants of the reaction vessel after the N2 and Cl2 react as completely as possible?
Answers
The correct option that represents the constants of the reaction vessel after the N₂ and Cl₂ react as completely as possible is A.
How to determine reaction vessel?The balanced chemical equation for the reaction is:
N₂(g) + 3Cl₂(g) → 2NCl₃(g)
This means that 1 mole of nitrogen gas and 3 moles of chlorine gas react to produce 2 moles of nitrogen trichloride gas.
The contents of the two vessels are:
Vessel 1: 1 mole of N₂
Vessel 2: 3 moles of Cl₂
When these two vessels are combined, the chlorine gas will react completely with the nitrogen gas to produce nitrogen trichloride gas. The remaining gas in the vessel will be nitrogen trichloride gas.
Which is:
[N₂] = 0 mol
[Cl₂] = 0 mol
[NCl₃] = 2 mol
Find out more on reaction vessel here: https://brainly.com/question/30057625
#SPJ1
Complete question:
11 Question 3 A =N O=CI N2 +3 Cl2 2 NC13 The contents of two vessels are combined, as shown. The balanced chemical equation for the reaction that occurs is also shown. Which of the following represents the contents of the reaction vessel after the N2 and Cl2 react as completely as possible?


We can use thin layer chromatography to check if Starbuck coffee has caffeine.
True
False
Answers
Answer:
false?
Explanation:
2. Describe how changes (mutations) to genes can result in changes to proteins.
Answers
Changes or mutations in genes can lead to changes in proteins through their impact on the genetic code and subsequent protein synthesis.
Genes carry the instructions necessary for the production of proteins, which are essential for various cellular functions. Mutations can occur spontaneously or due to factors such as environmental exposures, errors during DNA replication, or genetic predispositions.
Mutations can take various forms. Substitution mutations involve the replacement of a single nucleotide base with another, potentially altering the codon sequence in the gene. This change can result in the incorporation of a different amino acid during translation, leading to an altered protein structure and function.
Insertion or deletion mutations involve the addition or removal of nucleotides in the gene sequence. These mutations can disrupt the reading frame, causing a shift in the codon sequence downstream. As a consequence, the resulting protein can have an entirely different amino acid sequence, often resulting in a non-functional or severely impaired protein.
Mutations in regulatory regions of genes can also impact protein production. These regions control gene expression by influencing the binding of transcription factors. Alterations in these regulatory elements can lead to changes in the amount of protein produced, affecting cellular processes.
Overall, mutations in genes can result in changes to proteins by modifying the genetic code. These changes can affect protein structure, function, stability, and interaction with other molecules, ultimately impacting cellular processes, development, and disease susceptibility.
Know more about mutation here:
https://brainly.com/question/23030726
#SPJ8