Answers
Answer:
since neon has 10 electrons, phosphorous would contain 10 + 2 + 3 = 15 electrons, with 5 being valence electrons.
aka 15 dots in total, with 5 dots in the valence electron shell
Related Questions
Is salmon fortified or enriched?
Answers
What is the name of the functional group that is attached to this hydrocarbon?
H
H
• alkyl halide
alcohol
carbonyl
ketone

Answers
Two molecules having different sizes but the same functional groups will take part in chemical reactions. Functional groups are used in the field of organic chemistry. Here the given molecule has ketone functional group. The correct option is D.
What are functional groups?The substituent atoms or groups of atoms which are attached to specific molecules are defined as the functional groups. These moieties are responsible for the chemical reactions. The same functional group will experience comparable reactions regardless of the chemical in which it is present.
A ketone is a functional group which consists of a carbonyl carbon bound to an oxygen atom by a double bond and two alkyl or aryl groups. Ketones are usually represented as RCOR, where two R's can be any alkyl or aryl group. Their general formula is CₙH₂ₙO.
Lower ketones are soluble in water because they consist of the polar carbonyl group.
Thus the correct option is D.
To know more about functional group, visit;
https://brainly.com/question/29263610
#SPJ2
Zinc metal (Zn) will react with aqueous hydrochloric acid (HCI aq) to produce aqueous zinc chloride (ZnCl2 aq) and hydrogen gas (H2). Which of the following isthe complete, balanced equation for this reaction?1. 2 HCI (aq) + 2 Zn (s) > 2 H2 (g) + ZnCl2 (aq)2. HCI (aq) + Zn (s) > H2 (g) + ZnCl2 (ag)3. 2 HCI (aq) + Zn (s) › 2 H2 (g) + ZnCl2 (ag)4. 2 HCI (aq) + Zn (s) › H2 (g) + 2 ZnCl2 (ag)5. 2 HCI (aq) + Zn (s) › H2 (g) + ZnCl2 (ad)
Answers
Answer:
\(5\)Explanation:
Here, we want to get the equation of the reaction between Hydrochloric acid and Zinc metal
Zinc metal displaces the hydrogen from hydrochloric acid to form zinc chloride
We have the equation of reaction as:
\(2HCl_{(aq)}\text{ + Zn}_{(s)}\text{ }\rightarrow\text{ ZnCl}_{2(aq)}\text{ + H}_{2(g)}\)An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2. After performing the
experiment, 13.19 g of calcium carbonate, CaCO3, is produced. Calculate the percent yield of this reaction.
Answers
Answer:
Approximately \(81.84\%\).
Explanation:
Balanced equation for this reaction:
\({\rm Na_{2}CO_{3}}\, (aq) + {\rm CaCl_{2}} \, (aq) \to 2\; {\rm NaCl}\, (aq) + {\rm CaCO_{3}}\, (s)\).
Look up the relative atomic mass of elements in the limiting reactant, \(\rm CaCl_{2}\), as well as those in the product of interest, \(\rm CaCO_{3}\):
\(\rm Ca\): \(40.078\).\(\rm Cl\): \(35.45\).\(\rm C\): \(12.011\).\(\rm O\): \(15.999\).Calculate the formula mass for both the limiting reactant and the product of interest:
\(\begin{aligned}& M({\rm CaCl_{2}}) \\ &= (40.078 + 2 \times 35.45)\; {\rm g \cdot mol^{-1}} \\ &= 110.978\; \rm g \cdot mol^{-1}\end{aligned}\).
\(\begin{aligned}& M({\rm CaCO_{3}}) \\ &= (40.078 + 12.011 + 3 \times 15.999)\; {\rm g \cdot mol^{-1}} \\ &= 100.086\; \rm g \cdot mol^{-1}\end{aligned}\).
Calculate the quantity of the limiting reactant (\(\rm CaCl_{2}\)) available to this reaction:
\(\begin{aligned}n({\rm CaCl_{2}) &= \frac{m({\rm {CaCl_{2}})}}{M({\rm CaCl_{2}})} \\ &= \frac{17.87\; \rm g}{110.978\; \rm g \cdot mol^{-1}} \\ &\approx 0.161023\; \rm mol \end{aligned}\).
Refer to the balanced equation for this reaction. The coefficients of the limiting reactant (\(\rm CaCl_{2}\)) and the product (\({\rm CaCO_{3}}\)) are both \(1\). Thus:
\(\displaystyle \frac{n({\rm CaCO_{3}})}{n({\rm CaCl_{2}})} = 1\).
In other words, for every \(1\; \rm mol\) of \(\rm CaCl_{2}\) formula units that are consumed, \(1\; \rm mol\!\) of \(\rm CaCO_{3}\) formula units would (in theory) be produced. Thus, calculate the theoretical yield of \(\rm CaCO_{3}\!\) in this experiment:
\(\begin{aligned} & n(\text{${\rm CaCO_{3}}$, theoretical}) \\ =\; & n({\rm CaCl_{2}}) \cdot \frac{n({\rm CaCO_{3}})}{n({\rm CaCl_{2}})} \\ \approx \; & 0.161023\; {\rm mol} \times 1 \\ =\; & 0.161023\; \rm mol\end{aligned}\).
Calculate the theoretical yield of this experiment in terms of the mass of \(\rm CaCO_{3}\) expected to be produced:
\(\begin{aligned} & m(\text{${\rm CaCO_{3}}$, theoretical}) \\ = \; & n(\text{${\rm CaCO_{3}}$, theoretical}) \cdot M(({\rm CaCO_{3}}) \\ \approx \; & 0.161023\; {\rm mol} \times 100.086\; {\rm g \cdot mol^{-1}} \\ \approx \; & 16.1161\; \rm g \end{aligned}\).
Given that the actual yield in this question (in terms of the mass of \(\rm CaCO_{3}\)) is \(13.19\; \rm g\), calculate the percentage yield of this experiment:
\(\begin{aligned} & \text{percentage yield} \\ =\; & \frac{\text{actual yield}}{\text{theoretical yield}} \times 100\% \\ \approx \; & \frac{13.19\; {\rm g}}{16.1161\; {\rm g}} \times 100\% \\ \approx \; & 81.84\%\end{aligned}\).
During a class presentation, your classmate explains that plants perform photosynthesis and animals perform cellular respiration. In your own words, describe what these processes are and explain why your classmate’s statement is correct or incorrect.
Answers
My classmate's statement in incorrect because plants are also composed of cells.
All living things are composed of cells. A cell is the smallest unit of living organisms. Cells obtain energy via the process of cellular respiration. During cellular respiration, glucose is broken down to yield carbon dioxide and water.
In photosynthesis, carbon dioxide and water are combined to form glucose. The two processes are opposites of each other. Plants produce their own food via photosynthesis. Since plants are composed of cells, plant cells also undergo cellular respiration therefore my classmate's statement in incorrect.
Learn more: https://brainly.com/question/11324711
3. Explain what would happen to the digestion process if enzymes were not present. SC.6.L.14.5
Answers
An equilibrium mixture of N2, 02, and NO gases at 1500 K is determined to consist of
6.4 x101-3 mol/1 oF N2, 1.7 x 101-3 mol/ of 02 , and 1.1 × 10 ^-5 mol/1 of NO. What is the equilibrium constant for the system at this temperature?
Answers
The equilibrium constant for the system at this temperature is\(1.17 × 10^-31 mol^2/L^2\).
For the chemical equation:
N2(g) + O2(g) ⇌ 2NO(g)
The equilibrium mixture at a temperature of 1500 K is determined to contain 6.4 × 10^-3 mol/L of N2,\(1.7 × 10^-3\)mol/L of O2 and 1.1 × 10^-5 mol/L of NO. First, we need to calculate the concentration of N2 and O2 required to produce
1.1 × 10^-5 mol/L of NO:
2NO(g) = N2(g) + O2(g)
Given that there are 1.1 × 10^-5 mol/L of NO, the number of moles of N2 and O2 are equal since the stoichiometric ratio is 1:1. Therefore:
\(1.1 × 10^-5 mol/L\) = [N2][O2]Kc = \(([NO]^2)/([N2][O2])Kc\)= \((1.1 × 10^-5 mol/L)^2/(6.4 × 10^-3 mol/L)(1.7 × 10^-3 mol/L)Kc\) =
1.17 × 10^-31 mol^2/L^2.
for such more questions on equilibrium
https://brainly.com/question/5081082
#SPJ8
The rate of reaction of 0.030 g of magnesium ribbon with 1 M hydrochloric acid was studied at four different temperatures by measuring the time required for the magnesium metal to disappear. The following data was recorded: (4 points) Temperature 2°C 23 °C 40°C 53°C 56 Average Reaction Time (sec) 204 73 Average Reaction Rate (moles/sec) a. Calculate the number of moles of magnesium that reacted (2 points) b. Calculate and the average reaction rate for 2°C then complete the table above (2 points) c. Convert each temperature to kelvins (3 points) Temperature (°C) Temperature (K) 2°C 23°C 400 53 °C 75 °C Average Reaction Time (sec) 204 73 56 41 c. Plot the average reaction time versus temperature in the graph below. (10 points) e. Predict how long the reaction would take at 75 °C. (1 point) aation 10: Rate of Decomposition of Calcium Carbonate
Answers
Hydrogen gas is released and magnesium chloride is created when magnesium ribbon combines with hydrochloric acid.
The temperature of the test tube will change, there will be a faint fizzing of gas bubbles, and then the magnesium ribbon will dissolve into the water, turning it blue.
At four different temperatures, the time it took for 0.030 g of magnesium ribbon to react with 1 M hydrochloric acid was measured in order to determine the rate of reaction.
The following information was noted: Four points Temperature: 2°C, 23°C, 40°C, 53°C, and 56°C 204 seconds is the average reaction time. (moles/sec) Average Reaction Rate a. Decomposition of CaCO3 takes approximately one hour at 871 °C, resulting in a mass loss of approximately 44%, and approximately 24 hours at 641 °C.
To learn more about rate of reaction visit:
https://brainly.com/question/8592296
#SPJ4
If 39.5 g AlCl3 is produced, how many grams of HCl was used in the reaction
Answers
64.8 g of HCl was used in the reaction to produce 39.5 g of AlCl3.
What is Reaction?
A reaction is a process in which one or more substances are chemically transformed into one or more new substances. It involves the breaking of chemical bonds in the reactants and the formation of new bonds in the products. Reactions can occur spontaneously, as in the case of a burning match or rusting iron, or they may require the addition of energy, as in the case of photosynthesis or the combustion of fossil fuels.
The balanced chemical equation for the reaction between Al and HCl is:
2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)
From the equation, we see that 6 moles of HCl are required to produce 1 mole of AlCl3. We can use this ratio to calculate the moles of HCl required to produce 39.5 g of AlCl3.
First, we need to calculate the molar mass of AlCl3:
AlCl3: Al = 26.98 g/mol, Cl = 35.45 g/mol x 3 = 106.35 g/mol
Molar mass of AlCl3 = 26.98 + 106.35 = 133.33 g/mol
Now we can use the molar mass of AlCl3 to convert the mass of AlCl3 produced to moles:
39.5 g AlCl3 / 133.33 g/mol = 0.296 moles AlCl3
Finally, we can use the mole ratio from the balanced equation to calculate the moles of HCl:
6 moles HCl / 1 mole AlCl3 = x moles HCl / 0.296 moles AlCl3
x = 6 x 0.296 = 1.776 moles HCl
To convert this to grams, we can use the molar mass of HCl:
HCl: H = 1.01 g/mol, Cl = 35.45 g/mol
Molar mass of HCl = 1.01 + 35.45 = 36.46 g/mol
1.776 moles HCl x 36.46 g/mol = 64.8 g HCl
Learn more about Reaction from given link
https://brainly.com/question/25769000
#SPJ1
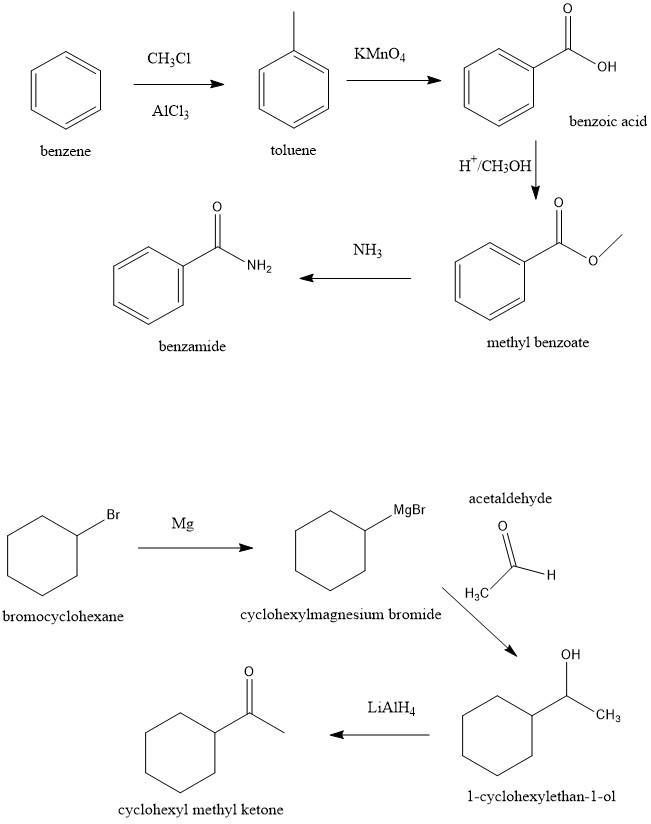
10. Show as many ways as you can think of: a) to make benzamide from benzene; b) to make cyclohexyl methyl ketone from bromocyclohexane;
Answers
Answer:
See explanation
Explanation:
a) Benzamide from benzene
For this synthesis, we have to start with the Friedel-Crafts reaction to produce Toluene. Then with a strong oxidant, we can produce benzoic acid. In the next step, we can use an esterification reaction to produce the methyl benzoate. Finally, we can use an acyl substitution reaction using ammonia to produce the benzamide.
b) From bromocyclohexane to cyclohexyl methyl ketone
In this case, we can start with a Grignard reaction. The first step is to produce the Grignard reagent with using magnesium. Then if we add acetaldehyde we can form an alcohol, 1-cyclohexylethan-1-ol. Finally, we can reduce the alcohol to produce cyclohexyl methyl ketone.
See figure 1
I hope it helps!
Can anyone please explain to me how that works?

Answers
Answer:
Explanation:
Have you done any Lewis diagrams? your picture of the drawings are kind like a Lewis diagram, showing the electrons filling of the outer shell. The outer shell balancing is what holds the atoms together in molecules. That's what you are showing in your drawing. How the atoms "stick" together.
Compare the number of moles of H ions to the number of moles of OH ions in the titration mixture when the HCL is exactly neutralized by the KOH
Answers
Answer:
When HCl (hydrochloric acid) and KOH (potassium hydroxide) are neutralized, they react to form water (H2O) and a salt (KCl). The balanced equation is:
HCl + KOH → KCl + H2O
In this reaction, one mole of HCl reacts with one mole of KOH to form one mole of water and one mole of KCl.
During titration of HCl with KOH, the point at which the reaction is complete is called the equivalence point. At the equivalence point, the moles of H+ ions and OH- ions are equal in the titration mixture.
Since one mole of HCl reacts with one mole of KOH, and H+ ions are present in HCl and OH- ions are present in KOH, the number of moles of H+ ions will be equal to the number of moles of OH- ions at the equivalence point.
Therefore, at the equivalence point, the number of moles of H+ ions will be equal to the number of moles of OH- ions in the titration mixture when HCl is exactly neutralized by KOH.
When the HCl is neutralized by KOH, the equivalence point is reached. During titration, the amount of HCl is determined using a basic solution of known concentration.
It is possible to calculate the amount of KOH required for complete neutralization if the initial concentration of the HCl solution is known. The balanced chemical equation for the reaction between HCl and KOH is:HCl + KOH → KCl + H2OThe stoichiometry of the reaction indicates that one mole of HCl reacts with one mole of KOH to produce one mole of H2O. Thus, the number of moles of H+ ions is equal to the number of moles of OH- ions when the equivalence point is reached.In an acid-base reaction, the number of moles of hydrogen ions (H+) produced by the acid is equal to the number of moles of hydroxide ions (OH-) produced by the base. When the HCl is exactly neutralized by the KOH, the number of moles of H+ ions is equal to the number of moles of OH- ions in the titration mixture.
This is due to the balanced chemical equation for the reaction, which shows that one mole of HCl reacts with one mole of KOH to produce one mole of water (H2O).Thus, at the equivalence point, the number of moles of H+ ions is equal to the number of moles of OH- ions. This is the point at which all of the HCl has reacted with the KOH. After the equivalence point, the excess KOH will react with the H2O to produce OH- ions, resulting in a basic solution.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
2223 25
TIME REMAINING
01:47:22
21
What can the arrow in a chemical reaction be translated to mean? Check all that apply.
O yields
Oaccompanied by
Dreact to form
Dadded to
Dexcept
Answers
The arrow in a chemical reaction can be translated as the following:
A. yields
C. react to form
which of the following solutions can act as a buffer solution? kcn/hcn na2so4/nahso4 nh3/nh4no3 nai/hi
Answers
KCN/ HCN forms a buffer solution. Thus, option
A is the correct answer.
The clear definition of a buffer solution in layman's language is a solution that contains a weak acid but a strong base. Furthermore, it is made up of two polar bonds that has polarities aligned in the same direction. It is used as an analytical reagent in chemistry.
The preparation of KCN(Potassium Cyanide) is obtained by treating HCN(Hydrogen Cyanide) and KOH( Potassium Hydroxide) then evaporation takes place leaving behind KCN. The chemical reaction involved in this is
HCN + KOH --> KCN + H₂O
KCN was a important source of alkali metals cyanides before the Castner's process.
To learn more about Potassium Cyanide,
https://brainly.com/question/7226735
#SPJ4
Which statements are NOT involved in boiling water? (check all the possible answers)
a.weakening intermolecular interactions
b.breaking covalent bonds
c.producing single atoms of H and O
d.breaking hydrogen bonds
Answers
There is no covalent bonds in pure H2O. Only hydrogen
How does the air temperature at the bottom of a mountain compare with the air temperature at the top of the mountain?
A. The air is colder at the bottom.
B. The air is warmer at the top.
C. The air temperature at the top is lower.
D. The air temperature is the same.
Answers
Explanation: C) the air temp. at the top is lower
How liters of HCLO3 will be produced from 10 litters of CLO2
Answers
The amount of \(HClO_3\) that will be produced from 10 liters of \(ClO_2\) would be 8.33 liters.
Stoichiometric problem\(HClO_3\) is produced from the reaction of \(ClO_2\) and \(H_2O\) according to the following equation:
\(6 ClO_2 + 3 H_2O --- > 5 HClO_3 + HCl\)
From the balanced equation of the reaction, 6 moles of \(ClO_2\) reacts to produce 5 moles of \(HClO_3\). Thus the mole ratio is 6:5.
Now, assuming the mole ratio also transcribes to the volume ratio and 10 liters of \(ClO_2\) is available for reaction. The amount of \(HClO_3\) that will be produced can be calculated from the volume ratio as follows:
6 moles \(ClO_2\) = 5 moles \(HClO_3\)
10 liters \(ClO_2\) = 5 x 10/6
= 8.33 liters
In other words, with 10 liters of \(ClO_2\), the volume of \(HClO_3\) that will be produced would be 8.33 liters.
More on stoichiometric problems can be found here: https://brainly.com/question/15047541
#SPJ1
a student mixed 20 grams of salt into a beaker with 200 milliliters of warm water. then, the student set the cup of saltwater on a windowsill undisturbed for one week. what changes did the student observe? include what happened when salt was mixed with warm water and what most likely happened to the saltwater after one week.
Answers
Answer:
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that held them together. After the salt compounds are pulled apart, the sodium and chloride atoms are surrounded by water molecules, as this diagram shows. Once this happens, the salt is dissolved, resulting in a homogeneous solution.
Explanation:
What type of bonding does Ir and Hg have?
Answers
Iridium forms metallic bonds, while mercury exhibits a combination of metallic and covalent bonding. These covalent interactions give rise to the low boiling point and weak intermolecular forces in liquid mercury.
Iridium (Ir) and mercury (Hg) exhibit different types of bonding based on their electronic configurations and properties.
Iridium is a transition metal belonging to Group 9 of the periodic table. It has a partially filled d-orbital in its atomic structure, which allows it to form metallic bonds. Metallic bonding occurs when the outer electrons of metal atoms are delocalized and form a "sea" of electrons that are free to move throughout the crystal lattice. This results in the characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility. Iridium forms metallic bonds with other iridium atoms, contributing to its solid, dense, and lustrous nature.
Mercury, on the other hand, is a unique element. It is a transition metal, but it exhibits characteristics of both metallic and covalent bonding. At room temperature, mercury exists as a liquid, which is highly unusual for a metal. This is because mercury atoms have a weak interatomic interaction, known as metallic bonding, similar to other metals. However, due to the presence of unpaired electrons in its 6s orbital, mercury can also form weak covalent bonds. These covalent interactions give rise to the low boiling point and weak intermolecular forces in liquid mercury.
In summary, iridium forms metallic bonds, while mercury exhibits a combination of metallic and covalent bonding.
For more question on bonds
https://brainly.com/question/29794367
#SPJ8
What effect does photosynthesis have on Earth’s atmosphere?
i need help who ever helps me will get a Brainliest
Photosynthesis removes both carbon dioxide and oxygen from the atmosphere.
Photosynthesis removes carbon dioxide from the atmosphere and adds oxygen to the atmosphere.
Photosynthesis adds both carbon dioxide and oxygen to the atmosphere.
Photosynthesis removes oxygen from the atmosphere and adds carbon dioxide to the atmosphere.
Answers
Photosynthesis removes carbon dioxide from the atmosphere and adds oxygen to the atmosphere.
Because plants basically breath Carbon and breathe out oxygen
hope this helps :)
What volume will 5.00 mol of an ideal gas occupy at 25.0 C. and 153 kPa of pressure?
Answers
Answer:
5.00 mol of an ideal gas will occupy 103.6 L at 25.0 C and 153 kPa of pressure.
Explanation:
Using the ideal gas law, PV=nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the temperature in Kelvin, we can solve for V.
First, we need to convert the temperature from Celsius to Kelvin by adding 273.15 K. Therefore, the temperature is 25.0 + 273.15 = 298.15 K.
Next, we can plug in the values we know:
PV = nRT
(153 kPa) V = (5.00 mol) (8.31 J/mol*K) (298.15 K)
Simplifying:
V = (5.00 mol) (8.31 J/mol*K) (298.15 K) / (153 kPa)
V = 103.6 L
Therefore, 5.00 mol of an ideal gas will occupy 103.6 L at 25.0 C and 153 kPa of pressure.
Calculate the enthalpy change for the reaction C2H4(g) + H2(g) -> C2H6(g) from the following data: Show your work.

Answers
The enthalpy change for the reaction \(C2H4(g) + H2(g) → C2H6(g)\) is -137.15 kJ/mol.
Given:C2H4(g) + H2(g) → C2H6(g)The enthalpy of formation of C2H6(g) is -84.68 kJ mol-1The enthalpy of formation of C2H4(g) is 52.47 kJ mol-1The enthalpy of formation of H2(g) is 0 kJ mol-1Hence, using Hess's Law, the enthalpy change for the reaction \(C2H4(g) + H2(g) → C2H6(g)\) can be calculated by considering the formation of reactants and products from their respective elements. It can be given as:
\($$C_2H_4 + H_2 → C_2H_6$$$$\Delta H = H_f(C_2H_6) - [H_f(C_2H_4) + H_f(H_2)]$$$$\Delta H = -84.68 - [52.47 + 0]$$$$\Delta H = -84.68 - 52.47$$$$\Delta H = -137.15 kJ/mol$$.\)
for more such questions on enthalpy
https://brainly.com/question/826577
#SPJ8
Hydrogen gas at a temperature of 22.0°C that is confined in a 5.00L cylinder exerts a pressure of 4.20atm. If the gas is released into a 10.0L reaction vessel at a temperature of 33.6°C, what will be the pressure inside the reaction vessel?
Answers
Answer:
THE NEW PRESSURE WHEN THE GAS IS RELEASED INTO A 10 L REACTION VESSEL AND AT 33.6 °C TEMPERATURE IS 2.18 atm.
Explanation:
The general gas equation will be used to calculate the new pressure.
P1V1 /T1 = P2 V2 / T2
P1 = 4.20 atm
V1 = 5 L = 5 dm3
T1 = 22°C = 22 + 273 K = 295 K
V2 = 10 L = 10 dm3
T2 = 33.6 °C = 33.6 + 273 K = 306.6 K
P2 =?
Rearranging the formula and making P2 the subject of the equation, we have;
P2 = P1V1 T2 / T1 V2
P2 = 4.2 * 5 * 306.6 / 295 * 10
P2 = 6438.6 / 2950
P2 = 2.18 atm.
So therefore, the pressure inside the reaction vessel when the gas is released into a 10 L reaction vessel and at a temperature of 33.6 °C is 2.18 atm.
Answer:
The pressure inside the reaction vessel is 2.18 atm
Explanation:
Step 1: Data given
The temperature = 22.0 °C = 295.15 K
Pressure = 4.20 atm
Volume = 5.0 L
Volume increased to 10.0 L
Temperature increased to 33.6 °C = 306.75K
Step 2: Calculate the new pressure
P1 * V1 / T1 = P2*V2 / T2
⇒with P1 = the initial pressure = 4.20 atm
⇒with V1 = the initial volume = 5.0 L
⇒with T1 = the initial temperature = 295.15 K
⇒with P2 = the new pressure = TO BE DETERMINED
⇒with V2 = the increased volume = 10.0L
⇒with T2 = the increased temperature = 306.75 K
(4.20 atm * 5.0 L) / 295.15 K = ( P2 * 10.0 L ) / 306.75 K
P2 = (4.20 * 5.0 L * 306.75 K) / (295.15 K * 10.0 L)
P2 = 2.18 atm
The pressure inside the reaction vessel is 2.18 atm
1) Wood has a density of 5.53g/cm3. What must the volume of 33.3 g of wood?
Answers
Answer:
6.021cm^3Explanation:
d=5.53 g/cm^3
m=33.3g
v=?
we know that,
d=m/v
or, v=m/d
or, v=33.3/5.53
•°• v=6.021cm^3
The volume of the wood is its mass divided by density. The volume of the 33.3 g wood with the density of 5.53 g/cm³ is 6.02 cm³.
What is density ?Density of a substance is the measure of its mass per unit volume. It describes how closely particles are packed. The density of a substance depends on the bond type, temperature and pressure.
If a substance is dense than water, it will sink on water. If it is less dense than water it will float on water. The ratio of the density of an object to that of water is called specific gravity.
Given that, density of wood = 5.53 g/cm³
mass = 33.3 g
volume = mass/density
= 33.3 g/ 5.53 g
= 6.02 cm³
Therefore, the volume of the wood is 6.02 cm³.
Find more on density:
https://brainly.com/question/29775886
#SPJ2
would you be able to help me?
Answers
Answer:
yes...
Explanation:
wha
Convert 150 grams of NaOH to particles of NaOH
Answers
150 grams of NaOH is approximately equal to 2.256 x 10^24 particles of NaOH.
To convert grams of NaOH to particles of NaOH, we need to use the concept of molar mass and Avogadro's number. The molar mass of NaOH is calculated by adding the atomic masses of sodium (Na), oxygen (O), and hydrogen (H) together. It can be determined as follows:
Na: 22.99 g/mol
O: 16.00 g/mol
H: 1.01 g/mol
Molar mass of NaOH = (22.99 g/mol) + (16.00 g/mol) + (1.01 g/mol) = 40.00 g/mol
Now, we can use the molar mass to convert grams of NaOH to moles. Since 1 mole contains Avogadro's number (approximately 6.022 x 10^23) particles, we can determine the number of particles as follows:
150 g NaOH * (1 mol NaOH / 40.00 g NaOH) * (6.022 x 10^23 particles / 1 mol NaOH) ≈ 2.256 x 10^24 particles
It's important to note that this calculation assumes the substance is pure NaOH and that the molar mass and Avogadro's number are accurate.
for more questions on particles
https://brainly.com/question/31213916
#SPJ11
What is the molecule shown below?н син н Н НТТТТТТн-с-с-с-с-с-с-н||||||H H CHOH Н НO A. OctaneOB. 4-propylpentaneOC. 2,3-dimethylhexaneOD. 2-pentylpropane

Answers
This molecule in the question presents 6 Carbons in its main chain, counting only the carbons in the middle, which makes it a Hexane molecule. Besides the main chain carbons, we also have two methyl groups linked to carbons 2 and 3, counting from left to right. Therefore the final name will be:
2,3-dimethylhexane. Letter C
if there are more products than reactants, does that mean there is an increase in the forward or backward reaction? And if there are more reactants that products, is there an increase in the forward or backward reaction?
Answers
Answer:
If there are more products than reactants, that means the reaction has shifted towards the left, which is the backward direction. If there are more reactants than products, that means the reaction has shifted towards the right, which is the forward direction.
According to today's Lesson Module Reading, which of the following are aqueous suspensions? Milk Soil Paint Jelly beans
Answers
Answer:
i think paint
Explanation:
determine if the following compounds are soluble (s) or insoluble (i) in what we based on the solubility chart
a. (NH4)2CO3
b.Fe(OH)2
c.CaOH
d. PbCl2
Answers
The solubility chart provides information about the solubility of various compounds in water. Here are the solubilities of the given compounds:
a. (NH₄)₂CO₃: According to the solubility chart, most carbonate (CO₃²⁻) salts are insoluble, except for those of Group 1 metals (alkali metals) and ammonium (NH₄⁺). Therefore, (NH₄)₂CO₃ is soluble.
b. Fe(OH)₂: Hydroxide (OH⁻) salts of transition metals, including iron (Fe), are generally insoluble, except for those of Group 1 metals and ammonium. Therefore, Fe(OH)₂ is insoluble.
c. Ca(OH)₂: Calcium hydroxide (Ca(OH)₂) is soluble. However, the given compound "CaOH" appears to be missing the subscript ₂, indicating two hydroxide ions. If it should be Ca(OH)₂, then it is soluble.
d. PbCl₂: According to the solubility chart, chloride (Cl⁻) salts, including lead chloride (PbCl₂), are generally soluble, except for those of silver (Ag⁺), lead (Pb²⁺), and mercury (Hg₂²⁺). Therefore, PbCl₂ is insoluble.
Learn more about solubility, here:
https://brainly.com/question/29857840
#SPJ1