How many significant figures are in the number 1.89 ' 10³?
A. 3
B. 1
C. 4
D. 5
E. 2
Answers
Answer:
A. 3
____________________________________________
What are significant figures?
Significant figures are used to establish the number which is presented in the form of digits. These digits carry a meaningful representation of numbers. The term significant digits are also used often instead of figures. We can identify the number of significant digits by counting all the values starting from the 1st non-zero digit located on the left. For example, 12.45 has four significant digits.
Learn more:
https://brainly.com/question/13386983
Have a WONDERFUL day! (:
Answer: A
Explanation:
1.89 ==> 1 + 8 + 9 ==> 3 sig figs ==> A
Related Questions
If the same large amount of heat is added to a 250 g piece of aluminum and a 150 g piece of aluminum, what will happen?
Answers
please vote me brainliest i really need it for i can do my work
How many moles of KC1 are in 1250 mL of 0.75 M KC1
Answers
The following formula can be used to determine how many moles of KC1 are present in 1250 mL of 0.75 M KC1: Molarity (M) is equal to the moles of solute per litre of solution.
In this instance, the volume of the solution is 1250 mL, and the molarity of KC1 is 0.75 M. The following formula can be used to determine how many moles of KC1 are present in 1250 mL of 0.75 M KC1: Molarity (M) times the number of litres in the solution equals 0.75 M times (1250 mL/1000 mL/L) or 0.9375 moles of KC1.
Consequently, 0.9375 moles of KC1 are present in 1250 mL of 0.75 M KC1. It is significant to remember that a solution's molarity is a measurement of the amount of a solute present in a given volume of the solution.
Learn more about molarity at:
https://brainly.com/question/8732513
#SPJ1
Seawater is 3.5% Sodium Chloride by mass. Sea Salt can contain about 10% water. How much water is removed from every Kilogram of Commercial Sea Salt?
Answers
The amount of water removed from every kilogram of commercial sea salt would be 27.57 kg.
Dimensional analysisSeawater contains 3.5% sodium chloride by mass. This means every 1 kg of seawater contains 0.035 kg of sodium chloride.
Sea salt contains about 10% water. This means every 1 kg of sea salt contains 0.1 kg of water.
In order to calculate the amount of water removed from every kilogram of commercial sea salt produced, we need to know the amount of seawater that will give 1 kilogram of sea salt.
We said every 1 kg of seawater contains 0.035 kg of salt. How many 0.035 kg can be found in 1kg?
1/0.035 = 28.57
This means 28.57 kg of seawater would need to be processed in order to have 1 kg of salt. Thus, the amount of water removed can be calculated as:
28.57 - 1 = 27.57 kg
In other words, about 27.57 kg of water is removed for every kilogram of commercial salt produced.
More on dimensional analysis can be found here: https://brainly.com/question/13078117
#SPJ1
I need help NOWW PLEASE HELP ME




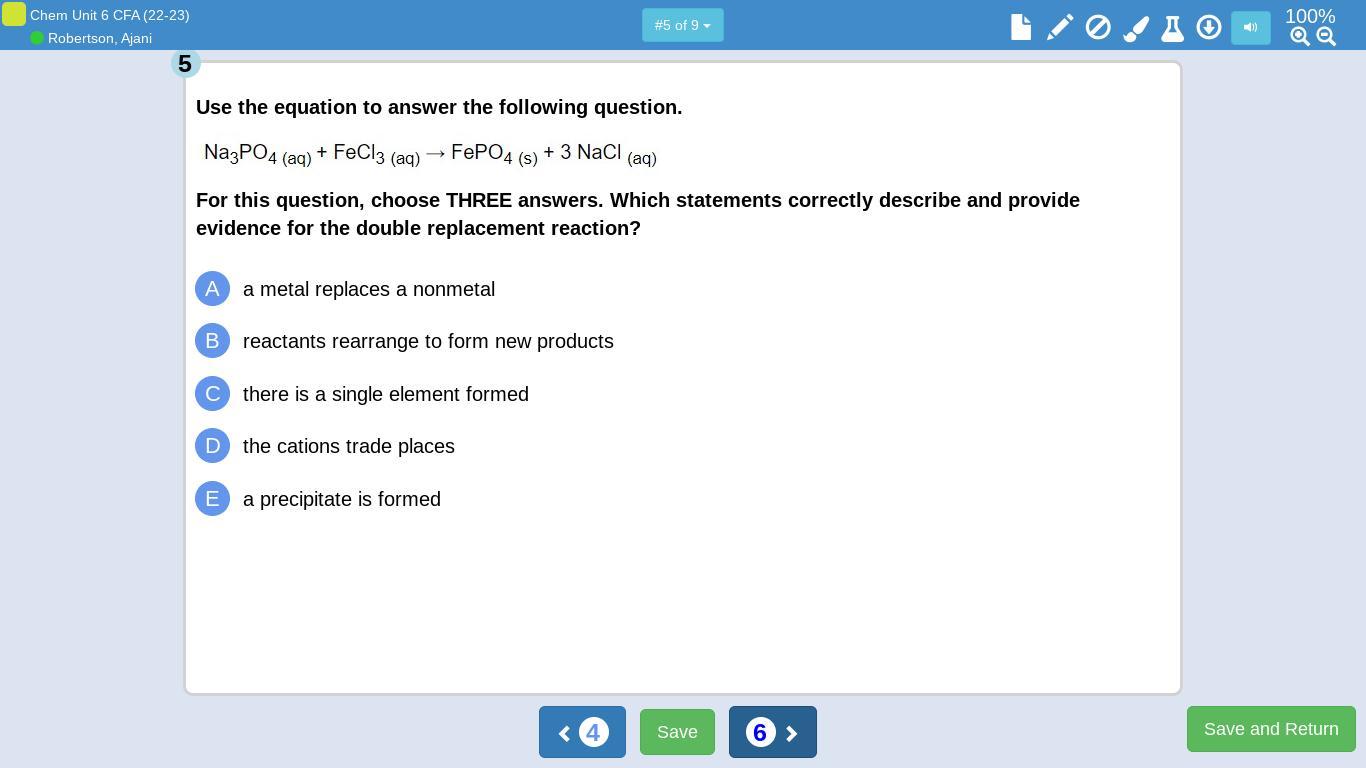
Answers
The reaction of Al₂S₃ with Li forms the displaced product Li₂S₃ and Al. The correct formula of the compound with X having one valence electron and Z with 6 valence electrons is XZ₂. The given reaction in the third image is a single replacement reaction and the reaction in the fourth image is double displacement. Options A, B and E are correct for the reaction in fifth image.
What is displacement reaction?In displacement reactions , an atom or group is displaced by other atom or group from a reactant. In double displacement reactions, two group or atoms are displaced between two reactants.
In the reaction between Al₂S₃ with Li forms the displaced product Li₂S₃ and Al. It is a single displacement reaction.
The element X with 1 valence electron will form a compound with Z having 6 valence electrons with the formula XZ₂. Z needs two electrons to attain octet. Thus two X provides one electron by each to Z.
The reaction of Na metal with HCl producing NaCl and hydrogen gas is a single displacement reaction. Whereas, the reaction between NaCl and MgF2 is double displacement because, both Cl and F atoms are displacing their position.
Sodium phosphate reacts with ferric chloride giving the ferric phosphate precipitate. Here the metals replaces nonmetals and the reaction involves formation of a precipitate. Thus, option A, B and E are correct.
To find more on displacement reaction, refer here:
https://brainly.com/question/3172917
#SPJ1
3. Which of the following are examples of chemical changes? Select all that apply.
-Water freezing
-Cookies baking
-Dry Ice subliming
-Wood burning
Answers
Answer:
wood burning and cookies baking
Explanation:
took the test <333
Answer:
Wood burning and cookies baking
Explanation:
15.a. Classify the following alkanes according to their increasing density. C₂Hs , C2H56, C11H24, C₂H6. C36H74
Answers
The order of alkanes according to their increasing density can be seen below:
C₂H5C₂H6C2H56C11H24C36H74What is Alkane?This is referred to a hydrocarbon with a single carbon to carbon bond and examples include butane etc.
Its density increases with increasing number of carbon and hydrogen atoms thereby making it the most appropriate choice.
Read more about Alkane here https://brainly.com/question/17040500
#SPJ1
A 5.0 balloon containing nitrogen gas is cooled from 25 degrees to 5°C. What is the final volume of the balloon?
Answers
Answer:
5.4
Explanation:
V1=5.0
T1=25+ 273=298
V2= z(unknown)
T2=5+273=278
V1/T1 =V2/T2
V2=V1T2/T1
=5*298/278
=5.4
What is the ratio of argon-40 to potassium-40 two half-lives
after the rock has formed?
Answers
The ratio of argon-40 to potassium-40 two half-lives after the rock has formed is 7:1.
The half-life of a radioactive compound tells us the time needed for half of the atoms of any compound to go through the radioactive decay.
We know that, half-life of potassium-40 is 1.25 billion years because this is how long it takes for half of the number of atoms which are present in the sample to decay to argon-40.
The half-life of potassium-40 which decays by the beta emission is 1.28 × 10⁹ years. The half-life of potassium-40 which decays by the positron emission is 1.19 × 10¹⁰ years.
40 K which naturally occurs decays to the stable 40 Ar which is 11.2%, It happens by the capture of electron and by positron emission. It gets decayed to stable 40 Ca (88.8%) by emission of negatrons. Half-life of 40 K 1.25 x 10⁹ years.
The dating method of potassium-argon is specifically useful in determining the Lava age.
To learn more about half-life,
brainly.com/question/14240789
#SPJ1
_____ are added to the soil to improve the fertility of the soil
Answers
Answer:
Fertilisers
And also organic compounds
A frozen popsicle is sitting outside in the sun. How will energy travel between the cold popsicle and the warm air around the popsicle?
Answers
Answer:
When the frozen popsicle sitting outside in the Sun is exposed to warm air, energy will travel between the popsicle and the warm air as follows;
1) Convection current
Heat will be gained by the frozen popsicle by coming into contact with fresh warm air as the air flows around the popsicle while the cooling of the warm air will cause the water vapor in the air to condense and form the visible mist
In turn the popsicle will gain heat resulting in melting of the ice
2) Conduction
As the some of the mist from the air settles on the popsicle, they share their heat resulting further melting of the popsicle
3) Radiation
Radiated heat energy from the Sun is absorbed by the popsicle resulting in the melting of the popsicle
Explanation:
How many electrons are removed if you ionize one mole of hydrogen using 13. 598V
Answers
By considering the concept of Faraday's constant and Avogadro's number we can say that one mole of hydrogen is ionized at 13.598V, removing around 6.022 × 10²³ electrons.
To determine the number of electrons removed when ionizing one mole of hydrogen using 13.598V, we can use the formula:
N = (1 mole) * (Avogadro's number)
where N represents the number of particles (in this case, electrons) in one mole of the substance.
Avogadro's number is approximately 6.022 × 10²³ particles/mol.
Therefore, the number of electrons removed can be calculated as:
N = (1 mole) * (6.022 × 10²³ particles/mol)
= 6.022 × 10²³ electrons
Thus, when ionizing one mole of hydrogen using 13.598V, approximately 6.022 × 10²³ electrons are removed.
To know more about the Ionization refer here :
https://brainly.com/question/1602374#
#SPJ11
oxalic acid, (90.03 g/mol), is a common, aqueous reagent in lab. after usage, it can be neutralized by koh. if you spent 28.05 ml of 0.103 m basic solution to neutralize 13.1 g of the aqueous acid solution, what is the mass percent of the acid in the original solution?
Answers
To calculate the mass percent of the acid in the original solution, you must first calculate the moles of acid used in the reaction. Since oxalic acid has a molar mass of 90.03 g/mol, 13.1 g of the acid is equivalent to 0.145 moles of acid.
Then, you can use the moles of acid to calculate the moles of base required to neutralize it. Since 28.05 ml of 0.103 M base was used, this is equivalent to 0.0288 moles of base. Since the reaction involves a 1:2 mole ratio of acid to base, the moles of acid used was twice the moles of base, or 0.145 moles.
Finally, you can calculate the mass percent of the acid in the original solution by dividing the mass of the acid used (13.1 g) by the total mass of the solution (13.1 g + 28.05 ml of 0.103 M base). This gives a mass percent of 32.2%.
Learn more about oxalic acid:
https://brainly.com/question/14897549
#SPJ4
Which mineral is commonly found in igneous rocks?
halite
feldspar
gypsum
talc
Answers
Answer:
Feldspars
Explanation:
Hope this helps! :) x
4. What trend in atomic radius occurs down a group on the periodic table?
rind on the periodic table?
Answers
Answer:Atomic radius gets bigger
Explanation:
Atomic radius bigger because not only do the atoms have more and more protons and neutrons, and thus more mass in general, there is also stronger shielding affect. Shielding affect is when electrons closer to the nucleus block the positive charge from reaching electrons farther from the nucleus, and thus those far electrons are not drawn towards the nucleus as strongly, and spread out more, increasing atomic radius.
what is a consquence of electron pair repulsion around an atom
Answers
Answer: Option c
Explanation:
Which stimulus is an example of an internal stimulus?
Responses
an artificial light that attracts insects
an artificial light that attracts insects
a sudden change of air temperature
a sudden change of air temperature
a decrease in oxygen levels in the blood during exercise
a decrease in oxygen levels in the blood during exercise
an increase in the amount of water available to a plant
Answers
a decrease in oxygen levels in the blood during exercise
an artificial light that attracts insects
stimulus is an example of an internal stimulus
What kinds of internal stimuli and reactions can you think of?Things like oxygen levels, blood sugar and pH levels, water levels, and internal temperature are examples of internal stimuli. If any of these factors becomes too high or too low, the body will react in order to reestablish homeostasis.
There are both internal and exterior stimuli. One illustration of external stimulation is how your body reacts to drugs. Vital sign alterations brought on by internal body changes are an example of internal stimulation.
learn more about homeostasis
https://brainly.com/question/24882789
#SPJ1
why is carbon iv oxide used in fire extinguishers
Answers
Answer:
Carbon dioxide extinguishes work by displacing oxygen, or taking away the oxygen element of the fire triangle. The carbon dioxide is also very cold as it comes out of the extinguisher, so it cools the fuel as well.
Given the nuclear equation:
232U + In → Ba + Kr + 3}n + energy
28.
State the type of nuclear reaction represented by the equation.

Answers
Answer:
Nuclear fission
Explanation:
Even though the equation is not too clear, however, we can see that the uranium nucleus was bombarded with a particle leading to its disintegration to yield other nuclides.
A nuclear fission reaction is one in which a heavier nuclide is bombarded with a particle (usually a neutron) leading to the disintegration of that nuclide into other nuclides with the release of tremendous energy. More neutrons are released in the process to continue the reaction. Hence, it is a chain reaction.
In 1927, a little-known Belgian priest named Georges Lemaître was the first to propose an expanding universe, based on Einstein’s theory of general relativity. Two years later, Edwin Hubble published a scatterplot of recessional velocity vs. galactic distance, shown above in the Velocity-Distance Relationship among Extra-Galactic Nebulae. This was the first observational evidence to support the hypothesis that grew into the Big Bang theory.
Describe how the evidence you have gathered in this Gizmo demonstrates that the universe is expanding and helps to confirm the Big Bang theory.
Answers
One of the pieces of evidence of the big bang theory where that celestial objects are scattered in the universe because of an explosion that supports the idea of the big bang.
What is the big bang?The big bang is how astronomers explain the way the universe began.
The evidence that I've gathered from the widget that demonstrates that the universe is expanding is that galaxies and other celestial objects are moving away from each other, which is also one of the evidence of the big bang theory where the celestial objects are scattered in the universe because of an explosion that supports the idea of the big bang.
Learn more about the big bang here:
https://brainly.com/question/18297161
#SPJ1
What happens when strong base is added to weak acid?
Answers
Answer:
The weak acid will give it's H+
Explanation:
If a strong base is added to a buffer, the weak acid will give up its H+ in order to transform the base (OH-) into water (H2O) and the conjugate base: HA + OH- → A- + H2O. Since the added OH- is consumed by this reaction, the pH will change only slightly.
4. How might the process of making paper from wood be changed to produce paper that is not acidic?
Answers
Answer:it is sterilized in the process
Explanation:
please help!! i am struggling with this. first correct answer gets brainliest!

Answers
Answer: \(1.25dm^3\) of unreacted oxygen is left.
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given volume}}{\text{Molar Volume}}\)
\(\text{Moles of} CO_2=\frac{3.60dm^3}{22.4dm^3}=0.161moles\)
\(\text{Moles of} O_2=\frac{7.25dm^3}{22.4dm^3}=0.324moles\)
\(C_3H_8(g)+5O_2(g)\rightarrow 3CO_2(g)+4H_2O(l)\)
According to stoichiometry :
3 moles of \(CO_2\) = 5 moles of \(O_2\)
Thus 0.161 moles of \(CO_2\) =\(\frac{5}{3}\times 0.161=0.268moles\) of \(O_2\)
moles of \(O_2\) left unreacted = (0.324-0.268) = 0.056
Volume of \(O_2\) left unreacted = \(moles\times {\text {Molar volume}}=0.056mol\times 22.4dm^3/mol=1.25dm^3\)
Thus \(1.25dm^3\) of unreacted oxygen is left.
What was the second most used type of material during the 1960 s? Select one:a) Semiconductors b)Composites c) Metals d) Polymers and Elastomers e) Ceramics and glasses
Answers
The correct answer to the given question is option c) Metals.
What were the materials used during the 1960s?
The materials used during the 1960s included metals, ceramics, glasses, semiconductors, polymers, and elastomers.
The 1960s were a period of significant technological advances.
A number of new and innovative materials emerged during this time that would shape the world for decades to com. Metals were the second most used material during the 1960s. During this time, there was significant demand for materials that could withstand high temperatures and pressures, as well as resist wear and tear and corrosion .Metals were used for a wide range of applications, including the construction of aircraft, automobiles, and other machinery.
They were also used in the production of electronic components, such as resistors and capacitors, and for the manufacture of a wide range of consumer goods, from kitchen utensils to jewellery. Metals remain a critical material in today's world, with a wide range of applications in industries ranging from aerospace and automotive to electronics and construction.
#SPJ11
Which factor causes a decrease in the rate of dissolution?
Answers
There are several factors that can cause a decrease in the rate of dissolution:
Decrease in temperature: As the temperature decreases, the kinetic energy of the particles decreases, and the rate of dissolution also decreases.
Increase in solute concentration: If the solution is already saturated with solute, then adding more solute will cause it to become supersaturated, which can cause a decrease in the rate of dissolution.
Increase in pressure: Increasing the pressure can force more solute into the solution, but it can also cause an increase in the solubility of the solute, which can cause a decrease in the rate of dissolution.
Decrease in surface area: If the solute is in the form of large particles, then breaking it down into smaller particles will increase the surface area available for dissolution and increase the rate of dissolution. Conversely, decreasing the surface area will decrease the rate of dissolution.
Formation of a precipitate: If the solute is capable of forming a precipitate in the solution, then the rate of dissolution may decrease as the solute is removed from the solution and deposited as a solid.
~~~Harsha~~~
Can someone help me
Determine the mass of 5.05 moles of magnesium (Mg)
Answers
Answer:
122.74024999999999
Explanation:
Answer:
\(\boxed {\boxed {\sf About \ 123 \ grams \ of \ Mg}}\)
Explanation:
To convert from moles to mass, we need to use the molar mass of the element. This can be found on the Periodic Table. It tells us the grams of magnesium in 1 mole of magnesium.
Magnesium (Mg): 24.305 g/molUse the molar mass as a fraction or ratio.
\(\frac{ 24.305 \ g \ Mg}{1 \ mol \ Mg}\)
Multiply by the given number of moles (5.05)
\(5.05 \ mol \ Mg *\frac{ 24.305 \ g \ Mg}{1 \ mol \ Mg}\)
The moles of magnesium will cancel each other out.
\(5.05 *\frac{ 24.305 \ g \ Mg}{1 }\)
The denominator of 1 can be ignored to create a simple multiplication problem.
\(5.05 *24.305 \ g \ Mg\)
\(122.74025 \ g \ Mg\)
The original measurement (5.05 moles) has 3 significant figures. Therefore we must round our answer to the 3 sig figs. For the number we calculated, that is the ones place.
The 7 in the tenth place tells us to round the 2 to a 3.
\(\approx 123 \ g \ Mg\)
There are about 123 grams of magnesium in 5.05 moles.
What is the atomic mass, in amu, of this atom?

Answers
Answer:
1) H - hydrogen - 1.100797
2) HE - helium - 4.00260
3) LI - lithum -6.941
Explanation:
On the periodic table the mass of carbon is reported as 12.01 amu. This is the average atomic mass of carbon. No single carbon atom has a mass of 12.01 amu, but in a handful of C atoms the average mass of the carbon atoms is 12.01 amu.
What is the molecular formula of a compound that has a mass of 276 and an empirical formula of NO?
Answers
Answer:
the molecular formula of the compound is N9O9.
Explanation:
To determine the molecular formula of the compound, we need to know the empirical formula mass of NO. The empirical formula of NO indicates that the compound contains one nitrogen atom and one oxygen atom.
The empirical formula mass of NO can be calculated as:
Empirical formula mass = atomic mass of N + atomic mass of O
Empirical formula mass = 14.01 + 16.00
Empirical formula mass = 30.01
The mass of the compound is given as 276. We can use this information along with the empirical formula mass to calculate the factor by which the empirical formula should be multiplied to obtain the molecular formula.
Factor = Molecular mass / Empirical formula mass
Factor = 276 / 30.01
Factor = 9.198
The molecular formula can be obtained by multiplying the empirical formula by this factor:
Molecular formula = Empirical formula x Factor
Molecular formula = NO x 9.198
Molecular formula = N9O9
Therefore, the molecular formula of the compound is N9O9.
how does science look at man
Answers

help meeeeeeeee pleaseeeeeeeeeee

Answers
Find the volume of 5.74 grams of NO2 (Hint: use two factors)
Answers
Answer:
1.2 cm^3
Explanation:
5.74 grams of a something occupies a volume of 1.2 cm^3 .