口
5. Which statement correctly describes HSO4- in the reaction represented by this equation?
HSO4- (aq) + H₂O(n) = H₂SO4(ng) + OH(aq)
* HSO4- is acting as a Brønsted-Lowry base because it is accepting a proton.
* HSO4- is acting as a Brønsted-Lowry acid because it is accepting a proton.
* HSO4- is acting as a Brønsted-Lowry acid because it is donating a proton.
* HSO4- is acting as a Brønsted-Lowry base because it is donating a proton.
Answers
Answer:
HSO₄⁻ is acting as a Brønsted-Lowry base because it is accepting a proton.
What is the Brønsted-Lowry Theory?
The Bronsted-Lowry theory states that acids act as proton donors, and bases act as proton acceptors; protons meaning hydrogen ions, since they only have 1 proton. This theory can be applied to practically any solvent.
HSO₄⁻(aq) + H₂O(l) ⇒ H₂SO₄(aq) + OH⁻(aq)
In the above equation:
the HSO₄⁻ accepts a proton from H₂O to form H₂SO₄the H₂O donates a proton to the HSO₄⁻ to form OH⁻Thus, the HSO₄⁻ is an base, and the H₂O is a acid.
Therefore, HSO₄⁻ is acting as a Brønsted-Lowry base because it is accepting a proton.
Related Questions
In life science, the decomposition of matter occurs after something dies. Write a sentence that uses the word decomposition.
Answers
Decomposition is the process of breakdown of complex organic matter into inorganic substances like carbon dioxide, water, and nutrients.
Decomposition or rot is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars, and mineral salts. The process is a part of the nutrient cycle and is essential for recycling the finite matter that occupies physical space in the biosphere.
Organisms that are responsible for the decomposition process are called decomposers. Most of the decomposers are microorganisms that cannot be seen through human eyes, like bacteria, some fungi, etc., but some decomposers are big enough that can be seen through human eyes.
learn more about decomposition at -
brainly.com/question/27746877
A molecule of (BLANK)
gas has a single covalent bond
between the two atoms that it's comprised of. This allows
each atom to attain the electron configuration of the noble
gas helium.
Answers
Answer:
Hydrogen
Explanation:
The noble gas helium is found in the first period in the periodic table. The only element that is also in that period is hydrogen.
Now, helium has two electrons in its outermost shell. It is the first member among the noble gases.
Each hydrogen atom has only one electron. Therefore, when two hydrogen atoms are joined by a singe covalent bond to form hydrogen gas, each hydrogen atom now has two electrons thereby attaining the electronic configuration of helium, the nearest noble gas to hydrogen.
a 50g sample of copper is at 25 c. of 1200 j of heat energy is added to the copper, what is its final temperature
Answers
The final temperature in the given situation is 87.015C. Using the heat capacity relation (Q=m* c*delta T).The copper tube expands by 1.7 × 10–5 of its length for every 1.8 degrees Fahrenheit.
How to solve?Step 1: Given data-
1. Mass of copper m = 50g= 0.05kg
2. Heat energy added Q = 1200 J
3. Specific heat of copper Scopper 4. The initial temp. is 25°C -387 J kg °C 1
Step 2: Find the rise in temperature using heat capacity relation- Let, final temperature be T. Then using heat capacity relation we can,
Q= m*c* delta T
On substituting values we get,
12000.05 x 387 x (T-25)
T= 87.015°C
Hence, the final temperature in the given situation is 87.015°C.
What is the temperature of copper wire?Copper melts at 1083°C. It has absolutely nothing to do with Minimum and Maximum temperature for a copper wire to work as a conductor. Both are determined by insulation and jacket materials. Insulation breaks down, if ambient temperature goes < 100°C.
Learn more about energy on:
https://brainly.com/question/8630757
#SPJ4
Consider the reaction A(g) +B(g) → C(g) +D(g) for which AH° = +85.0 kJ and AS--66.0 J/K. You may assume that AH° and Asº do not change with temperature. What can you conclude about this reaction? a) It is spontaneous at low T, but nonspontaneous at high T. b) The reverse reaction is spontaneous at all temperatures. c) It is nonspontaneous at low l but spontaneous at high T. d) It is spontaneous at all temperatures. multiple choice: a b c d
Answers
Consider the reaction A(g) +B(g) → C(g) +D(g) for which AH° = +85.0 kJ and AS--66.0 J/K. You may assume that AH° and Asº do not change with temperature. This reaction is: nonspontaneous at low l but spontaneous at high T. The correct option is (c).
Considering the reaction A(g) + B(g) → C(g) + D(g) with ΔH° = +85.0 kJ and ΔS° = -66.0 J/K, we can use the Gibbs free energy equation to determine the spontaneity of the reaction at different temperatures. The Gibbs free energy equation is:
ΔG° = ΔH° - TΔS°
In this case, ΔH° is positive, and
ΔS° is negative.
To determine the spontaneity, we can analyze the signs of ΔG° at different temperatures:
a) At low T, the TΔS° term will be small and negative, making ΔG° positive, which indicates a nonspontaneous reaction.
b) At high T, the TΔS° term will be large and negative, potentially making ΔG° negative, which indicates a spontaneous reaction.
Thus, the reaction is nonspontaneous at low temperatures but spontaneous at high temperatures, which corresponds to option (c).
To know more about "Gibbs free energy" refer here:
https://brainly.com/question/9179942#
#SPJ11
What’s the answer for this?

Answers
Answer:
it will fall then rise and return to ×
iodine is one of many compounds that can be used as a stain for tlc, however, there are many stains that can be used to identify specific functional groups. looking online, propose a good tlc stain to identify dehydroabietylamine on a tlc plate?
Answers
The iodine staining technique is one of the methods that can be used as a stain for TLC, however, there are many stains that can be used to identify specific functional groups.
The iodine staining technique allows us to carry abound a marked version of owe 'TLC' sun rather than having to pencil sketch ow spots in the UV views. Fore compounds might not even appear under Uv light, making otter visualization ted. such as iodine staining necessary.One of the first techniques for displaying organic chemicals was to stain a TLC plate with iodine vapor. The discovery that iodine has a strong affinity for both unsaturated and aromatic molecules forms the basis for this theory.Iodine is one of the substances that can be used as a TLC stain, however, there are numerous stains that can be used to identify particular functional groups. Iodine sublimes and interacts with the compounds on a produced TLC plate when the chamber is sealed, creating yellow-brown patches.Learn more about TLC stain at:
brainly.com/question/17562109
#SPJ4
The angle formed by the nuclei of two surrounding atoms with the nucleus of the central atom in a structure is called a(n) ________angle. The value predicted for such an angle using the VSEPR theory would be 180 degrees, based upon geometry alone. This is referred to as the___________ bond angle. In practice, this value often deviates from the predicted value for various reasons.
Answers
The bond angle obtained based on the geometry of the molecule alone is known as the predicted bond angle.
When a compound is formed, the less electronegative atom in the compound is called the central atom in the molecule. The angle formed by the nuclei of two surrounding atoms with the nucleus of the central atom in a structure is called a bond angle.
The bond angle is usually based on the geometry of the molecule. The expected geometry can be deviated for certain reasons such as the presence of lone pairs on the central atom in the molecule. The bond angle obtained based on the geometry of the molecule alone is known as the predicted bond angle.
Learn more about bond angle: https://brainly.com/question/6179102
Which is the BEST question to find out whether an object has the right property for you to carry it in your backpack?
What does the object sound like?
What does the object taste like?
What is the color of the object?
What is the size of the object?
Answers
Answer: what is the size of the object
Explanation: then again though, i wouldn't carry something in my backpack that didn't taste good...
Which equation below is an example of a single-replacement reaction?
A.6CO2(g) + 6H2O(l) → C6H12O6(aq) + 6O2(g)
B.H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
C.Ca(OH)2(s) Δ→ CaO(s)+ H2O(l)
D.Zn(s) + 2HCl(aq)→ ZnCl2(aq) + H2(g)
Answers
The equation that depicts a single replacement reaction would be: Zn(s) + 2HCl(aq)→ ZnCl2(aq) + H2(g)
Single replacement reactionsThey are also known as single displacement reactions.
They are reactions in which one element takes the place of another in a compound. That is, one element replaces another in a compound.
Looking at all the reactions from A - D, one can see that the only reaction that exemplifies a single replacement reaction is D.
Here, Zn replaced H in HCl.
More on single replacement reactions can be found here: https://brainly.com/question/13328989
Calculate the frequency of yellow light with a wavelength of 580 x 10–9 m.
Answers
Answer:
5791
Explanation:
Easy peasy lemon squeazy
Which is an example of a beneficial mutation? ANSWER AND ILL GIVE BRAINLY POINTS !!!!!! HELP ASAP !
Answers
Answer:
Some humans are randomly immune to malaria or have increased bone density due to random beneficial mutations.
Explanation:
Answer: one that changes the color of a rabbit, allowing it to hide from predators.
Explanation:Hey give me brainliest award need it
6. In which of the following will the bulb glow?
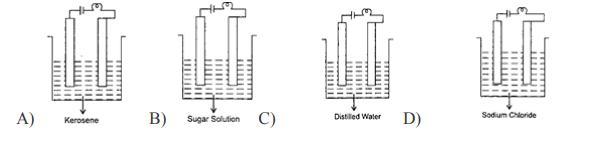
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later
If the mass of aluminum is 1. 80 g and iodine is 2. 30 g how much of the excess reagent remains after the reaction
2Al + 3I2 > 2AlI3
Answers
Total, 0.77 g of I2 is the amount of the excess reagent that remains after the reaction.
To determine the excess reagent remaining, we first need to find the limiting reagent.
The balanced equation tells us that 2 moles of Al react with 3 moles of I₂ to form 2 moles of AlI₃. We can use this information to calculate the theoretical yield of AlI3 based on the amount of each reactant;
moles of Al = 1.80 g / 26.98 g/mol = 0.067 moles
moles of I₂ = 2.30 g / 253.81 g/mol = 0.009 moles
Since the stoichiometry of the reaction is 2:3 for Al and I₂ , respectively, we can see that I₂ is the limiting reagent. Thus, all of the Al will react, while some of the I₂ will be left over.
The amount of AlI₃ that can be formed from the limiting reagent (I2) is:
moles of AlI₃ = 0.009 moles I₂ × (2 moles AlI₃ / 3 moles I₂ )
= 0.006 moles AlI₃
The mass of AlI₃ that can be formed is;
mass of AlI₃ = 0.006 moles × 407.82 g/mol
= 2.47 g
Since we know that only 2.30 g of I₂ was present initially, we can calculate the amount of excess I₂ remaining after the reaction;
excess I₂ = 2.30 g - (0.009 moles I₂ × 253.81 g/mol)
= 0.77 g
Therefore, 0.77 g of reagent that remains after the reaction.
To know more about theoretical yield here
https://brainly.com/question/14966377
#SPJ4
what does Le châteliers principle state?
Answers
Hope this helps!
the equilibrium constant for the reaction c6h5cooh(aq) ch3coo–(aq) c6h5coo–(aq) ch3cooh(aq) is 3.6 at 25°c. if ka for ch3cooh is 1.8 × 10–5, what is the acid dissociation constant for c6h5cooh?
Answers
The equilibrium constant between C6H5COOH and \(CH_3COO^-\) is 3.6 at \(25^0C\). Given that the acid dissociation constant (Ka) for \(CH_3COOH\) is \(1.8 * 10^–^5\), we need to determine the acid dissociation constant for \(C_6H_5COOH\).
The equilibrium constant (K) relates to the concentrations of the reactants and products at equilibrium. For the given reaction, the equilibrium constant (K) is expressed as \([C_6H_5COO^-][CH_3COOH]/[C_6H_5COOH][CH_3COO^-]\) and is equal to 3.6 at \(25^0C\).
To find the acid dissociation constant (Ka) for \(C_6H_5COOH\), we can use the relationship between K and Ka. Since the reaction involves the acid \(C_6H_5COOH\), we can write the equation as follows:
\(C_6H_5COOH =C_6H_5COO^-+ H^+\)
The equilibrium constant (K) for this reaction is equal to\([C_6H_5COO^-][H^+]/[C_6H_5COOH]\). We can relate this to the acid dissociation constant (Ka) by noting that [\(H^+\)] is equivalent to the concentration of the dissociated acid, and [\(C_6H_5COOH\)] is the initial concentration of the acid.
Since the acid dissociation constant (Ka) for \(CH_3COOH\) is given as \(1.8 * 10^-^5\), we can set up the following equation:
\(1.8 *10^-^5 = [C_6H_5COO^-][H^+]/[C_6H_5COOH]\)
Knowing that the equilibrium constant (K) is 3.6, we substitute the appropriate values into the equation:
\(3.6 = [C_6H_5COO^-][CH_3COOH]/[C_6H_5COOH][CH_3COO^-]\)
By rearranging the equation and substituting the given values, we can solve for the acid dissociation constant (Ka) of \(C_6H_5COOH\).
Learn more about equilibrium constant here:
https://brainly.com/question/29809185
#SPJ11
write the noble gas configuration for carbon
Answers
Answer:
[He] 2s2 2p2
Explanation:
2. How many moles of salt are present in 1.5L of a 5.OM salt water solution?
Answers
Answer:
There are 7.5 moles of salt
Explanation:
5.0M means that in every liter of solution, there are 5 moles of salt. So, 1.5L of solution times 5 moles per liter equals 7.5 moles
what is molecular formula of Ozone?
Answers
Answer:
the molecular formula of Ozone is O_3.
Answer: O₃
Explanation:
The chemical formula of ozone is O3. In the earth's stratosphere, ozone is formed from a two-step reactive process. First, sunlight breaks apart an oxygen molecule (O2 you'll recall) into two oxygen atoms. In the second step, these oxygen atoms collide with another oxygen atom to make ozone.
Name the following compounds:

Answers
1. The name of the compound is 2-methyl-2-hexene
2. The name of the compound is 4-ethyl-3,5-dimethylnonane
3. The name of the compound is 4-methyl-2-heptyne
4. The name of the compound is 5-propyldecane
How do i determine the name of the compound?The name of each compound given in the question can be obtained as follow:
For question 1
Locate the longest continuous carbon chain. In this case it is carbon 6. Hence, the parent name is hexene since a double bond is involvedIdentify the substituent groups attached. In this case the substituent group attached is methyl, CH₃ Give the substituent the lowest possible count while considering the double bond. In this case, CH₃ is located at carbon 2Combine the above to obtain the IUPAC name for the compound.Thus, the IUPAC name for the compound is: 2-methyl-2-hexene
For question 2
Locate the longest continuous carbon chain. In this case it is carbon 7. Hence, the parent name is nonaneIdentify the substituent groups attached. In this case the substituent groups attached are methyl, CH₃ and ethyl, CH₂CH₃ Give the substituents the lowest possible count. In this case, the two CH₃ are located at carbon 3 and 5 while the CH₂CH₃ is located at carbon 4Combine the above to obtain the IUPAC name for the compound.Thus, the IUPAC name for the compound is: 4-ethyl-3,5-dimethylnonane
For question 3
Locate the longest continuous carbon chain. In this case it is carbon 7. Hence, the parent name is heptyne since a triple bond is involvedIdentify the substituent group attached. In this case the substituent group attached is methyl, CH₃ Give the substituent the lowest possible count while considering the triple bond. In this case, CH₃ is located at carbon 4Combine the above to obtain the IUPAC name for the compound.Thus, the IUPAC name for the compound is: 4-methyl-2-heptyne
For question 4
Locate the longest continuous carbon chain. In this case it is carbon 10. Hence, the parent name is decaneIdentify the substituent groups attached. In this case the substituent group attached is propyl, CH₂CH₃CH₃ Give the substituent the lowest possible count. In this case, CH₂CH₃CH₃ is located at carbon 5Combine the above to obtain the IUPAC name for the compound.Thus, the IUPAC name for the compound is: 5-propyldecane
Learn more about IUPAC name:
https://brainly.com/question/23881815
#SPJ1
draw lewis structures for the ethylene molecule ( c2h4 ), the chloromethane molecule ( c2hcl ), and the acetaldehyde molecule ( ch3cho ), and then answer the questions that follow.
Answers
The lewis structures of ethylene, chloromethane, and acetaldehyde are shown in the attached diagram below.
What is the lewis electron dot structure?Lewis structures can be described as diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can possibly draw for any covalently bonded molecule and coordination compounds.
A lewis electron dot structure can be used to represent the total number of bonds, the different bonding atoms, and the lone pairs left in the atoms in the molecule.
Solid lines are used to show the bond between atoms that are bonded directly to one another and lone pairs of electrons are represented as dot pairs and are placed next to the atoms.
Learn more about the lewis dot diagram, here:
brainly.com/question/14091821
#SPJ1



How does an electron move from the ground state to an excited state?It absorbs energy.It becomes a neutron.It releases energy.It becomes a proton.
Answers
In this question, we have to determine the way in which an electron move from a ground state to an excited state, and the way this occurs is by absorbing energy, when the right amount of energy hits the electron, this electron will jump to a higher energy level. Therefore the answer will be the 1st option
Which description is not a property of an acid?
Sour taste
Corrosive
Slimy feel
Dissolves metals
Answers
Answer: C - Slimy feel.
Explanation:
I was deciding between C or A, but acids are in fact sour in taste, corrosive & they dissolve metals, so C is the answer.
hope this helps ! ! <3
What does the alpha isomer of a carbohydrate have? A) The anomeric OH on the same side of the CH2OH group B) The anomeric OH on the opposite side of the CH2OH group C) No anomeric OH group
Answers
The alpha isomer of a carbohydrate has the anomeric OH on the same side of the \(CH_{2}OH\) group (option A).
What is the structure of alpha isomer of carbohydrate?
The alpha isomer of a carbohydrate has A) The anomeric OH on the same side of the \(CH_{2}OH\) group. This configuration is what differentiates it from the beta isomer, which has the anomeric OH on the opposite side of the \(CH_{2}OH\) group. This means that the hydroxyl group (-OH) attached to the anomeric carbon (the carbon that is bonded to two oxygen atoms) is on the same side as the \(CH_{2}OH\) group in the cyclic structure of the carbohydrate. The beta isomer, on the other hand, has the anomeric OH on the opposite side of the \(CH_{2}OH\) group (option B). If there is no anomeric OH group, then it is not a cyclic carbohydrate and is instead an open-chain form (option C).
To know more about Carbohydrates:
https://brainly.com/question/14614055
#SPJ11
6. Neutral Atom of Carbon
Atomic Number =
Number protons =
Number of electrons =
Atomic Mass = (rounded)
Number of neutrons =
Number of Energy Levels =
Is the last energy level full?
+-AT-4Arial
233 BI 2
Answers
Answer:
6
6
2,4
12.01070 unit
6 neutron
2 energy levels
1) The hydrocarbon C15 H32 burns to form carbon dioxide and water. Write the equation for the reaction.
2) How would you test the products when C15 H32 burns to show that carbon dioxide had been formed.
Answers
\(\\ \tt\hookrightarrow C_{15}H_{32}+23O_2\longrightarrow 15CO_2+16H_2O\)
In order to check carbon dioxide we need to pass the gas through lime water then the lime water turn cloudy or milkyBecause it forms calcium carbonate\(\\ \tt\hookrightarrow CO_2+CaO=CaCO_3\)
1) The balanced equation for the combustion of C15H32 (pentadecane) is:
C15H32 + 23O2 → 15CO2 + 16H2O
2) To test for the presence of carbon dioxide (CO2) in the products of the combustion of C15H32, one simple way is to bubble the gas through limewater (aqueous calcium hydroxide solution). When carbon dioxide is passed through limewater, it reacts with the calcium hydroxide to form a white precipitate of calcium carbonate according to the following chemical equation:
Ca(OH)2 + CO2 → CaCO3 + H2O
The formation of a white precipitate indicates the presence of carbon dioxide in the gas. Another way to test for the presence of carbon dioxide is to use a pH indicator, such as universal indicator or litmus paper. When carbon dioxide dissolves in water, it forms a weak acid called carbonic acid. This can be detected by observing a change in the color of the pH indicator. Carbon dioxide will turn universal indicator yellow or litmus paper red, indicating an acidic solution.
Can someone please help me with this, ill give you a heart, a 5 star rating, and the brainliest answer!

Answers
A weather map of Chicago with a high pressure system and warm front. Based on the weather map, what might the upcoming weather be like in Chicago? Warm, dry, clear skies Warm, humid, possible thunderstorms Cold, dry, clear skies Cool, humid, possible thunderstorms.
Answers
A weather map of Chicago with a high pressure system and warm front, so the upcoming weather be Warm, dry, clear skies in Chicago.
What is high pressure of air?High pressure of air tries to compress the gas from upper atmosphere to lower atmosphere.
When any gas has high pressure then it sinks towards the land from the upper atmosphere, and at the upper part air gets cool and form water vapor. When air comes towards the land then, it becomes warm and dry as it doesn't participate in the formation of precipitate. Due to which we are able to see clear sky at the dry days.
Hence, option (1) is correct i.e. Warm, dry, clear skies are the upcoming weather.
To know more about pressure of air, visit the below link:
https://brainly.com/question/441281
Answer:
A. Warm, dry, clear skies
( Hope this helps ) <3 ( Give person above brainliest )

Which is true and explain?
a. An increased number of protons and electrons increases the attraction between them.
b. An increased number of protons and electrons decreases the attraction between them.
C. An increased number of protons and electrons has no effect on the attraction between them
Answers
Answer:
a. An increased number of protons and electrons increases the attraction between them.
Explanation:
The positively charged protons in the nucleus attract the negatively charged electrons. As the number of protons in the nucleus increases, the electronegativity or attraction will increase.
Compare 2,3-pentanediol and 2,4-pentanediol with respect to the number of stereoisomers possible for each. Which ones are chiral? Which are achiral?
Answers
Both molecules have two chiral centers, one at each point of attachment for a hydroxyl group.
What are stereoisomers?According to a general definition, stereoisomers are isomers with the same composition (i.e., the same components), but different orientations in space. Enantiomers and diastereomers are the two different types of stereoisomers.
X = \(2^{n}\), where n is the total number of stereogenic atoms in the molecule, is the formula for calculating the maximum number of stereoisomers. The maximum number of stereoisomers can be accurately determined by the formula X = \(2^{n}\), but in cases of great symmetry, it cannot determine the actual number.
Both molecules have two chiral centers, one at each point of attachment for a hydroxyl group. It is not possible to have a stereoisomer of 2,3-pentanediol with a plane of symmetry, while there is one stereoisomer of 2,4-pentanediol with a plane of symmetry. The plane of symmetry reduces the possible number of stereoisomers.
To know more about chiral centers refer to:
https://brainly.com/question/9522537
#SPJ1
An irregularly-shaped piece of aluminum (Al) has a mass of 70.5 grams. What is the volume in cm3 of this piece of aluminum if its density is 2.70 g/cm3?
Answers
The volume of aluminum will be "26.12 cm³".
Aluminum:A translucent bright silvery metal from Periodic Group 13 of such periodic chart, is a Aluminum.
Aluminum is found in numerous everyday products as as kitchenware and timepieces. It is being used throughout the building industry to make doors, window frames, cables, as well as roofs.
According to the question,
Mass of substance = 70.5 gDensity of substance = 2.70 g/cm³We know,
The volume of substance:
= \(\frac{Mass}{Density}\)
By putting the values,
= \(\frac{70.5}{2.70}\)
= \(26.12\) cm³
Thus the response above is right.
Find out more information about volume here:
https://brainly.com/question/1529039
