Answers
The correct statement in regards to nuclear fusion are 1) Only in the sun's core does fusion take place because of the necessary temperatures.
2) The three types of neutrinos oscillate one after the other.
3) It takes very high temperatures for nuclei to merge.
The Sun and other stars are powered by nuclear fusion reactions. A heavier nucleus is created when two light nuclei fuse together in a fusion reaction. Because the mass of the resulting single nucleus is smaller than the combined mass of the two initial nuclei, the process produces energy. Remaining mass is converted to energy.
Controlled nuclear fission reactions and chemical reactions like burning coal, oil, or gas each produce roughly four million times as much energy as controlled atom fusion (at equal mass).
For more information on nuclear fusion kindly visit to
https://brainly.com/question/12701636
#SPJ4
Related Questions
Giúp mình 2 câu này với ạ
1, Pha 500ml dung dịch HCl 10% (w/v) từ dung dịch 37% (w/w) d=1.19 g/ml
2, Cách pha như thế nào để nhận được 200g dung dịch HCl 25% (w/w) từ dung dịch HCL 37% (d=1.19 g/ml)
Answers
Answer:it is wrong answer
Explanation:estro man
A) What is the wavelength of electromagnetic radiation that has a frequency of 5.97 x 10MHz? (1 MHz = 1 x 10 Hz)
Answers
Answer:
rrgffffffffffrrrddhgf
Explanation:
trrdfgfxfff
A 13.58 g sample of a compound contains 8.67 g of iron, Fe, 1.60 g of phosphorus, P, and oxygen, O. Calculate the empirical
formula for the compound.
Answers
Answer:
\(Fe_3PO_4\)
Explanation:
To do this, we find the moles of each element. We get around 0.155 moles of Fe, 0.051 moles of P, and 0.206 moles of O. We then divide each one by the smallest one (which is 0.051 moles of P). We then get 3 for Fe, 1 for P, and 4 for O. This correlates to the empirical formula of the compound.
The empirical formula for the compound is \(Fe_3PO_4\) if a 13.58 g sample of a compound contains 8.67 g of iron, Fe, 1.60 g of phosphorus, P, and oxygen, O.
What is the empirical formula?
An empirical formula tells us the relative ratios of different atoms in a compound.
We need to calculate the number of moles:
Given data:
Mass of iron - 8.67 g
Mass of phosphorus -1.60 g
Mass of oxygen -3.31 g
Moles of iron - \(\frac{mass}{molar \;mass}\)
Moles of iron - \(\frac{8.67 g}{56 g/mol}\)
0.15 mole
Moles of phosphorus \(-\frac{mass}{molar \;mass}\)
Moles of phosphorus - \(\frac{1.60 g}{31 g/mol}\)
0.051 moles
Moles of oxygen -\(\frac{mass}{molar \;mass}\)
Moles of oxygen - \(\frac{3.31 g}{16 g/mol}\)
0.20 moles
Dividing each mole using the smallest number that is divided by 0.051 moles.
Fe:P:O :: 3:1:4
The empirical formula for the compound is \(Fe_3PO_4\).
Learn more about empirical formula here:
brainly.com/question/14044066
#SPJ1
(a) Define
(a) chromatography
(b) TLC
(c) retention factor (Rf)
(b) Identify two factors that affect the retention factor values.
(c) Give the equation that can be used for the calculation of Rf.
Answers
Chromatography is a laboratory technique used to separate and analyze mixtures of molecules based on their physical and chemical properties, such as their size, charge, and polarity. The technique involves passing a mixture of molecules through a stationary phase, such as a column or a thin layer of material, which separates the molecules based on their interactions with the stationary phase.
What is TLC about?TLC stands for Thin Layer Chromatography, which is a specific type of chromatography that uses a thin layer of material, such as silica gel or alumina, as the stationary phase.
Retention factor (Rf) is a measure of how far a molecule travels through the stationary phase relative to the distance traveled by the solvent front. Rf is calculated by dividing the distance traveled by the compound by the distance traveled by the solvent front.
Two factors that can affect retention factor values are the polarity of the stationary phase and the polarity of the mobile phase. The polarity of the stationary phase affects how strongly the molecules in the mixture interact with the stationary phase, while the polarity of the mobile phase affects how easily the molecules can move through the stationary phase.
In conclusion, equation that can be used for the calculation of Rf is:
Rf = distance traveled by compound / distance traveled by solvent front
Learn more about retention factor from
https://brainly.com/question/24731831
#SPJ1
Which factor does not influence whether a substance will be a liquid at room temperature and normal atmospheric pressure?
O molecular mass
O surface tension
O molecular shape
O strength of intermolecular forces
20pts
Answers
Surface tension
Hope it helps
Which best describes the activation energy on the graph below?
Potential Energy
Reaction Progress
3
OA. The vertical difference between 1 and 3
B. The vertical difference between 1 and 2
OC. The difference between the x-axis and 2
OD. The vertical difference between 2 and 3

Answers
The statement that best describes the activation energy on the graph below is the vertical difference between 1 and 2 (option B).
What is activation energy?Activation energy is the energy required to initiate a reaction. For example, the flame from the fuse of a firecracker provides a small initial amount of energy, after which the explosive reaction proceeds by itself, releasing a considerably larger quantity of energy.
According to this question, a reaction proceeds from 1 to 3. The activation energy needed for the reaction to be initiated is the vertical line that extends to the peak of 2.
Therefore, it can be said that the statement that best describes the activation energy on the graph below is the vertical difference between 1 and 2.
Learn more about activation energy at: https://brainly.com/question/11334504
#SPJ1
Which reaction would you find in a radioisotope thermal generator? O © ²H+ ²H → He+ n 2 ○ 325U + √n → 14¹Ba + Kr+3 n 92 92 56 36 O 338 Pu 234U+He²+ → 94 92 H+ ²H → 2He o -
Answers
The equation found in the radioisotope thermal generator is option C
What is a radio isotope thermal generator?A device called a radioisotope thermoelectric generator (RTG) makes use of the heat produced when radioactive isotopes decay.
Using a thermocouple, a device that transforms heat into electrical energy, the heat generated by radioactive decay is transformed into electricity. Numerous spacecraft, such as the Voyager probes, the Cassini-Huygens mission to Saturn, and the Viking missions to Mars, have been propelled by RTGs.
Learn more about radio isotope:https://brainly.com/question/2293396
#SPJ1

Which of the following is NOT a way planets heat up during formation.
Radioactivity
Differentiation
Convection
Accretion
Answers
Hope this helps
Consider the neutralization reaction
2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq)
A 0.105 L sample of an unknown HNO3 solution required 41.1 mL
of 0.150 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3 solution?
Concentration:
Answers
The concentration of the HNO3 solution is 0.117 M.
To solve this problem, we can use the balanced chemical equation to determine the mole ratio of HNO3 to Ba(OH)2:
2HNO3(aq) + Ba(OH)2(aq) ⟶ 2H2O(l) + Ba(NO3)2(aq)
From the equation, we see that 2 moles of HNO3 react with 1 mole of Ba(OH)2 to produce 2 moles of H2O and 1 mole of Ba(NO3)2. Therefore, the moles of HNO3 in the unknown solution can be calculated from the volume and concentration of Ba(OH)2 used:
moles of Ba(OH)2 = concentration × volume = 0.150 M × 0.0411 L = 0.006165 mo
moles of HNO3 = 2 × moles of Ba(OH)2 = 2 × 0.006165 mol = 0.01233 mol
Finally, we can calculate the concentration of the HNO3 solution:
concentration = moles / volume = 0.01233 mol / 0.105 L = 0.117 M
For more question on concentration click on
https://brainly.com/question/26255204
#SPJ11
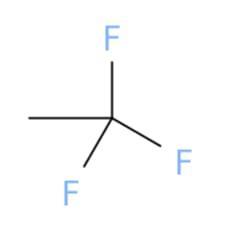
Select the correct structure that
corresponds to the name.
1,1,1-trifluoroethane

Answers
The correct chemical structure that corresponds to 1,1,1-trifluoroethane is (a).
What is 1,1,1-trifluoroethane?
A chemical structure is a spatial arrangement of atoms in a molecule. It determines the molecular geometry and when necessary the electronic chemistry as well .1,1,1-Trifluoroethane or simply known as trifluoroethane is Hydrofluorocarbon (HFC) compound that is colourless and highly inflammable gas with ether like odour. One method of preparation of 1,1,1-Trifluoroethane is by fluorination of 1-chloro-1,1-difluoroethane in the presence of hydrofluoric acid. The chemical formula for 1,1,1-Trifluoroethane is \(C__{2} } H_{3} F_{3}\). The high stability of it's chemical structure because of being heavier than air makes it a greenhouse gas with high infrared absorbent power. It can be used as a propellant or refrigerant and in cleaning of electrical equipments.
Learn more about 1,1,1-trifluoroethane here:
https://brainly.com/question/1390779
#SPJ1
Convert a length of 22.0 m to inches
Answers
Answer:
866.142 inches
Explanation:
By formula
A sample of ammonia, NH3, has a mass of 78.25 g. Calculate the number of ammonia molecules in the sample.
number of molecules:
Answers
There are approximately \(2.76 * 10^{24\) ammonia molecules in the given sample.
To calculate the number of ammonia molecules in the sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia \((NH_3)\) can be calculated by adding up the atomic masses of nitrogen (N) and hydrogen (H):
Molar mass of \(NH_3\) = (1 x atomic mass of N) + (3 x atomic mass of H)
= (1 x 14.01 g/mol) + (3 x 1.01 g/mol)
= 14.01 g/mol + 3.03 g/mol
= 17.04 g/mol
Now, we can calculate the number of moles of ammonia in the sample using the formula:
Number of moles = Mass of the sample / Molar mass
Number of moles = 78.25 g / 17.04 g/mol
≈ 4.5865 mol (rounded to four decimal places)
Finally, we can use Avogadro's number, which represents the number of particles (atoms, molecules, etc.) in one mole of a substance. Avogadro's number is approximately \(6.022 * 10^{23\) particles/mol.
Number of ammonia molecules = Number of moles x Avogadro's number
Number of ammonia molecules ≈ 4.5865 mol x (\(6.022 * 10^{23\) molecules/mol)
≈ \(2.76 * 10^{24\) molecules (rounded to two significant figures)
Therefore, the provided sample contains roughly \(2.76 * 10^{24\) ammonia molecules.
Learn more about moles on:
https://brainly.com/question/24748125
The number of ammonia molecules in the sample is approximately 2.764 x \(10^{24}\) molecules.
To calculate the number of ammonia molecules in a given sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia (NH3) is calculated as follows:
Molar mass of N = 14.01 g/mol
Molar mass of H = 1.01 g/mol
Total molar mass of NH3 = 14.01 g/mol + (3 * 1.01 g/mol) = 17.03 g/mol
Now, we can calculate the number of moles of ammonia in the sample:
Number of moles = Mass of sample / Molar mass of NH3
Number of moles = 78.25 g / 17.03 g/mol = 4.594 moles
Next, we use Avogadro's number, which states that there are 6.022 x \(10^{23}\) molecules in one mole of a substance.
Number of molecules = Number of moles * Avogadro's number
Number of molecules = 4.594 moles * 6.022 x \(10^{23}\) molecules/mol = 2.764 x \(10^{24}\) molecules
Therefore, there are approximately 2.764 x \(10^{24}\) ammonia molecules in the given sample of 78.25 g.
Know more about Avogadro's number here:
https://brainly.com/question/1513182
#SPJ8
We have an aqueous solution that contains 35% (by mass) of a hypothetical solute Z. The formula weight of the solute Z is 118 g/mol. The density of the solution is observed to be 1.4 g/mL. What is the molarity of Z in this solution
Answers
Answer:
4.15 M
Explanation:
In order to find the molarity of a stock solution of Z, we use the formula;
Co = 10pd/M
where;
p = percentage by mass of Z
d = density of Z
M= molar mass of Z
Substituting values;
Co= 10 * 35 * 1.4/118
C0= 4.15 M
Which action demonstrates a CHEMICAL CHANGE?
A- Long hair is cut and dried.
B- A wooden pencil is sharpened and breaks
C- An ice cube melts and becomes a clear liquid.
D- An iron nail becomes orange and flaky on the surface.
Answers
Who has course hero?I really need the “lion king…Ecology science.” Answer key so I can print it.It wont let me print mines.
Answers
Note that the above prompt on Ecology draws it's analysis form a well known story which have been told visually called "The Lion King".
The answers are:
1) Biotic Factors, simply put are living things
2 examples of things from Lion King Introduction are:
3) Abiotic factors are Non -living things.
4) Examples from the introduction are:
MountainWaterDirt5) the symbiotic relationship is called: commensalism.
What is commensalism?Long-term biological interactions known as commensalism occur when individuals of one species benefit while those of the other species suffer neither advantages nor harm.
Ecology which is the study of the environment, allows a person to comprehend how different types of creatures coexist in various kinds of physical settings.
Learn more about Ecology at:
https://brainly.com/question/30429252
#SPJ1
Full Question:
Although part of your question is missing, you might be referring to this full question:
1) What is Biotic Factors
2) List three biotic factors from the Lion King introduction
3) What is Abiotic factors?
4) List three Abiotic factors from the Lion King introduction
5) The bird riding on the tusks of the elephant feed on insects the elephant stirs up. What kind of symbiotic relationship exists between the two?
Which is for which? Here is the image to my question. Please help god bless.

Answers
Answer:
1 - Gravitational.
2 - Normal
3 - Tension
4 - Frictional
5 - Centripetal
Explanation:
1. If you drop something, gravity pulls it down to the Earth, So falling towards the earth is gravity.
2. Pushing back on another object is normal, Newton's law: Every action has an equal and opposite reaction.
3. When two forces are pulled on opposite sides, the object must stretch which creates tension. Think of a rubber band. If it is pulled more than the object can stretch, it will tear. Tensile strength refers to how much pulling force an object can withstand before it tears.
4. When objects or molecules rub against other objects or molecules they create friction.
5. Last two options go together.
Which aquatic organism would be least likely to survive in a <7 pH environment?
protozoa
worms
algae
bacteria
Answers
Drag each item to the correct location to indicate whether it is a greenhouse gas or a non-greenhouse gas.
Answers
It is a greenhouse gas. The greenhouse effect is caused by the absorption by the surface of the Earth and re-absorption by the atmosphere of the greenhouse gases (CH₄, CO₂, NO, CFCs ) in the environment.
What is the greenhouse effect?
The greenhouse effect is a natural process by which certain gases in the Earth's atmosphere, known as greenhouse gases, trap heat from the sun and prevent it from escaping into space. The greenhouse gases, which include carbon dioxide, methane, water vapor, and others, act like a blanket around the Earth, absorbing and re-emitting infrared radiation that would otherwise be lost to space. This process helps to keep the Earth's temperature within a range that is suitable for supporting life. However, human activities such as burning fossil fuels have increased the concentration of greenhouse gases in the atmosphere, leading to an enhanced greenhouse effect and causing the Earth's temperature to rise, a phenomenon known as global warming.
To know more about greenhouse effect, visit:
https://brainly.com/question/13706708
#SPJ1
The complete question and the labelled answer respectively, is as follows:


A student planned to make copper sulfate crystals from excess copper oxide and dilute sulfuric acid.
The equation for the reaction is:
CuO(s) + H,SO (aq) -, CuSO (aq) + H20(1)
This is the method used.
1. Add 25 cm° of dilute sulfuric acid to a conical flask.
2. Gently warm the dilute sulfuric acid.
3. Add excess copper oxide to the dilute sulfuric acid.
4. Stir the mixture.
5. Heat to evaporate all the water from the mixture.
Suggest two improvements to the method
Explain why each improvement is needed.
A student plans a method to prepare pure crystals of copper sulfate.
The student's method is:
1. Add one spatula of calcium carbonate to dilute hydrochloric acid in a beaker.
2. When the fizzing stops, heat the solution with a Bunsen burner until all the liquid is gone.
The method contains several errors and does not produce copper sulfate crystals.
Explain the improvements the student should make to the method so that pure crystals of copper sulfate are produced.

Answers
The student's method for preparing pure crystals of copper sulfate contains errors and does not produce the desired outcome.
Use copper oxide instead of calcium carbonate: The student should add copper oxide (CuO) to the hydrochloric acid instead of calcium carbonate. Copper oxide reacts with hydrochloric acid to form copper chloride, which can then be converted to copper sulfate through a subsequent reaction with sulfuric acid.
Add sulfuric acid to the copper chloride solution: After the copper chloride solution is formed, the student should add sulfuric acid to it. This reaction between copper chloride and sulfuric acid will yield copper sulfate and hydrochloric acid. The student should ensure that the correct stoichiometric ratio is maintained to maximize the yield of copper sulfate crystals.
Crystal formation: The student should allow the solution to cool slowly after the reaction with sulfuric acid. This promotes the formation of larger, well-defined copper sulfate crystals.
Filtration and drying: Once the crystals have formed, the student should filter the solution to separate the solid crystals from the remaining liquid. The filtered crystals should then be thoroughly dried to remove any remaining water, resulting in pure copper sulfate crystals.
By following these improvements, the student can obtain pure crystals of copper sulfate.
For more such questions on copper sulfate visit:
https://brainly.com/question/17439051
#SPJ8
Select all the correct answers
When two generalizations can be made based on what you know about cycles of matter in a closed system?
New matter is added, and old matter is destroyed.
Matter changes its physical form, allowing it to return to its original state.
The amount of matter within the system remains the same
Matter and energy can cross the boundaries of the system.
The cycle has a well-defined starting and Stopping point

Answers
Answer:
A
Explanation:
What is an extensive property that can be calculated?
Answers
Answer: The property which depends on the quantity of the substance is called an extensive property. The free energy change for a reaction (Δ G) depends on the quantity of the substance and is therefore an extensive property. It shows the additive nature. The extensive property Δ G is easily calculated from the formula, ΔG = -nFE cell.
Explanation:
Identify each of the following compounds as an alcohol, a phenol, or an ether. Drag the appropriate items to their respective bins.
Answers
The functional group in alcohol is a hydroxyl group (-OH), in phenol, it is a hydroxyl group attached to a benzene ring, and in ether, it is an oxygen atom (-O-) between two alkyl or aryl groups.
Given that the question asks to identify each of the following compounds as an alcohol, a phenol, or an ether. The question seems to be incomplete as there are no options or compounds mentioned. However, given below is a general explanation of the three compounds -Alcohol: Alcohol is a compound that contains a hydroxyl functional group (-OH) that is attached to a carbon atom. The hydroxyl group in alcohol makes it polar and thus, able to form hydrogen bonds. Alcohol is used as a solvent, fuel, and disinfectant. Phenol: Phenol is an aromatic compound that contains a hydroxyl group (-OH) attached to a benzene ring. It is used in the production of detergents, plastics, and pharmaceuticals. Phenol is used as a disinfectant and anesthetic. Ether: Ether is an organic compound that contains an oxygen atom between two alkyl or aryl groups. It is a colorless, volatile, and highly flammable liquid that is used as a solvent and as a starting material in the synthesis of organic compounds. To identify a compound as an alcohol, phenol, or ether, we need to examine the functional groups in the molecule.
To learn more about Functional group :
https://brainly.com/question/696051
#SPJ11
The following liquids are poured into a glass jar. Which is in the correct order, from top to bottom, how the liquids would form based on density? *
Answers
Answer:thing chang into different thing
Explanation:
What kind of graph shows how data change over time, with no lines
connecting the data points?
Answers
Answer:
Bar Graphs
Explanation:
Answer: Scatterplot
Explanation:

How many molecules of sugar C6H1206 are in a mole?
Answers
Answer:
i.e. mass of 1 mole of glucose, C6H12O6 = (6 × 12.01 + 12 × 1.01 + 6 × 16.00) g = 180.18 g (using atomic weight data to 2 decimals) 1 mole of carbon atoms weighs 12.01 g and there are 6 moles of C atoms in 1 mole of glucose, so the mass of carbon in 1 mole of glucose = 6 × 12.01 g = 72.06 g.
Modeling Nuclear changes

Answers
Answer:
can someone please do this and put the answers below !!!!
Explanation:
HELP ASAP PLEASE ANSWER BOTH OF THESE QUESTIONS! I WILL GIVE YOU BRAINELIST IF YOU ANSWER IT!


Answers
Answer:
because it absorbs all colours expect for red,
Explanation: making the red light bounce into your eyes, therefore seeing the colour red
Question 2: The wave transfers its energy to the mineral, I think, thats my best guess
Is there more than one possible model that could be inferred from Rutherford’s data?
Answers
Rutherford proposed the nuclear model of atom. The only one model which can be inferred from this model is nucleus with protons and revolving electrons around.
What is Rutherford model?Rutherford proposed some aspects of atomic structure based on his gold foil experiment. He discovered that the alpha ray is scattering from foil by the repulsion it experienced from the metal.
This results lead to the discovery of positively charged particles in atom latter called as protons. Rutherford proposed that theses protons are located inside the nucleus and the electrons are revolving around the nucleus.
Latter Niels Bohr his student interpreted the nuclear model of Rutherford with quantum mechanics and theory of max planck and he proposed the equations for the determination of energy and momentum of electrons and the radius of atom.
Therefore, no other inferences except the presence of protons and revolving electrons can be obtained from Rutherford's model of atom.
To find more about nuclear model, refer the link below:
https://brainly.com/question/18521318
#SPJ2
C17H19NO3 compound, what is the percent by mass of carbon
Answers
Answer:
71.58%
Explanation:
Find the Mr of C₁₇H₁₉NO₃ :
(12 × 17) + 19 + 14 + (16 × 3) = 285
Mr of carbon in C₁₇H₁₉NO₃ :
12 × 17 = 204
Percent by mass of carbon = \(\frac{204}{285}\) × 100 = 71.58%
The element magnesium, Mg, has three common isotopes:24Mg, 25Mg, and 26Mg. The difference between these three isotopes is Question 7 options: the number of neutrons. the number of electrons. the number of protons. the number of protons and electrons. their physical state.
Answers
The difference between all three Magnesium, Mg isotopes is in; the number of neutrons.
What is isotopy?Isotopy is a property of elements in which case the element has two or more types of atoms that have the same atomic number and hence, same position in the periodic table, but differ in nucleon numbers due to different numbers of neutrons in their nuclei.
Ultimately, the distinctive feature between the three isotopes is in their number of neutronsRead more on isotopes;
https://brainly.com/question/14220416