Balance each of the following examples of heterogeneous equilibria and write each reaction quotient, Qc:(c) S₈(s) + F₂(g) ⇄ SF₆(g)
Answers
When we balance this equation
S₈(s) + F₂(g) ⇄ SF₆(g)
We will get
S₈(s) + 24F₂(g) ⇄ 8SF₆(g)
Balancing this equation
S₈(s) + F₂(g) ⇄ SF₆(g)
We have to balance the number of S
S₈(s) + F₂(g) ⇄ 8SF₆(g)
We have to balance the number of F
S₈(s) + 24F₂(g) ⇄ 8SF₆(g)
We will get balanced equation
S₈(s) + 24F₂(g) ⇄ 8SF₆(g)
The reaction quotient will be
Qc = [product] / [reactant]
Qc = [SF₆(g)] / [ S₈(s) + F₂(g)]
To learn more about reaction quotient click the given link
https://brainly.com/question/17144280
#SPJ4
Related Questions
what do scientists use to classify substances that are either an acid or a base?
Answers
Answer:
They use the pH scale
Explanation:
The strong acid and strong base has high rate constant of dissociation. Weak acid and base are the one whose rate constant for the dissociation is low, they do not dissociate readily in water. Scientist use pH scale to classify substances that are either an acid or a base.
What are acid and base?Acid is a solution which releases H⁺ hydrogen ion when dissolved in water. Base are the solution which releases hydroxide ion OH⁻ ion when dissolved in water. . At room temperature pH scale is between 0 to 14. pH of neutral solution is 7.
The pH of acid is between 0-7 on pH scale. acid turns blue litmus paper to red. Acids have sour taste and corrosive nature. Mathematically the concentration of hydrogen ion can be calculated using this formula
Mathematically,
pH=-log[H⁺]
Therefore, scientist use pH scale to classify substances that are either an acid or a base.
To know more about acid and bases, here:
https://brainly.com/question/27228111
#SPJ2
It takes 547 kJ to remove one mole of electrons from the atoms at the surface of a solid metal.
What is the maximum wavelength of light capable of doing this?
Answers
According to the relation of variables in the electromagnetic spectrum the maximum wavelength of light is 36.3 ×10\(^-\)³¹ m.
What is electromagnetic spectrum ?The electromagnetic spectrum consists of electromagnetic radiation consists of waves made up of electromagnetic field which are capable of propogating through space and carry the radiant electromagnetic energy.
The radiation are composed of electromagnetic waves which are synchronized oscillations of electric and magnetic fields . They are created due to change which is periodic in electric as well as magnetic fields.
In the given problem,energy is related to wavelength by the formula, λ=hc/E,λ=6.626×10\(^-34\)×3×10⁸/547×1000=36.3×10\(^-31\) m.
Thus, the maximum wavelength of light is 36.3×10\(^-31\) m.
Learn more about electromagnetic spectrum,here:
https://brainly.com/question/23727978
#SPJ1
-You are studying a sample of an element. You determine that the element's
atom has 5 protons, 5 electrons, and a mass number of 11. You determine
this is a boron atom. How many neutrons does it have?
Answers
Answer:
6
Explanation:
The mass number is the sum of the number of protons and the number of neutrons so 11-5=6
Students are completing an investigation on types of heat transfer. For one part of the investigation, they place their hand on a cool window and feel the difference in thermal energy. When they are touching the glass, what type of heat transfer are they experiencing?
a .Conduction
b. Convection
c. Radiation
Answers
If you have a recommendation of 3 K2O/acre, how much Potash do
you need to purchase for 11 acres?
Answers
Potash is commonly measured in pounds or kilograms, so you may need to convert the units accordingly. Additionally, it's always recommended to consult with a professional or refer to specific guidelines for accurate recommendations
To determine the amount of Potash needed to purchase for 11 acres with a recommendation of \(3 K2O/acre\), you can follow these steps:
1. Calculate the total amount of Potash needed for 11 acres:
\(3 K2O/acre * 11 acres = 33 K2O\)
2. Convert K2O to Potash (K2O is the chemical formula for Potash):
\(33 K2O * (1 Potash / 1 K2O) = 33 Potash\)
Therefore, you would need to purchase 33 units of Potash for 11 acres with a recommendation of\(3 K2O/acre\).
It's important to note that the specific unit of measurement for Potash was not provided in the question
Remember to double-check your calculations and units to ensure accuracy.
To know more about accuracy visit:
https://brainly.com/question/28482209
#SPJ11
A _______ is a substance used during electrolysis that splits apart and is attracted to an oppositely charged electrode
Answers
Answer:
electrolyte
Explanation:
What is the process of old crust sinking back into the mantle? *
a. earthquake
b. transform boundary
c. seafloor spreading
d. subduction
Answers
Answer:
dddddddddddddd
ddddddddddddddddd
Give the formula for tetrasilicon heptabromide.
Answers
The formula for tetrasilicon heptabromide is Si₄Br₇.
What is chemical formula?Chemical formula, also called molecular formula is a notation indicating the number of atoms of each element present in a compound.
The chemical formula of a substance reveals the type and number of elements in the compound. According to this question, a chemical compound named tetrasilicon heptabromide is given.
Based on the nomenclature of the compound, it can be said that it is made up of four atoms of silicon and seven atoms of bromine, hence, the chemical formula is given thus: Si₄Br₇.
Learn more about chemical formula at: https://brainly.com/question/29031056
#SPJ1
One of the reasons plants are important to us is because they reduce the amount of carbon dioxide in the atmosphere and increase the amount of oxygen, which we need to breathe. Explain how this statement relates to the chemical equation for photosynthesis.
Answers
Answer:
Photosynthesis is a phenomenon in which green plants such as algae, fungus etc containing chlorophyll use sunlight as a source of energy to convert carbon dioxide and water present in the atmosphere to form glucose which is used as plant food and oxygen which is liberated into the atmosphere for breathing.
The chemical equation for photosynthesis is:
\(6CO_2+6H_2O\rightarrow C_6H_{12}O_6+6O_2\)
where carbon dioxide and water are reactants and glucose and oxygen are products formed.
How many carbon atoms are in each mole of calcium carbonate?
Answers
Answer:
5 atoms are in each molecule of calcium carbonate
Draw the molecules or ions on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including charges where needed. The single bond is active by default.
Answers
Open the molecular drawing software of your choice: Molecular drawing software allows you to create and manipulate molecules or ions using a computer.
Some popular examples of molecular drawing software include ChemDraw, ChemSketch, and MarvinSketch. You can download and install these programs on your computer or use online versions if available.
Select the "Tools" toolbar and choose the bond type you want to use: Bonds are the connections between atoms in a molecule or ion. The "Tools" toolbar in molecular drawing software contains options to select the bond type you want to use, such as single, double, or triple bonds. You can also choose to draw dashed or solid wedges to indicate stereochemistry.
Select the "Atoms" toolbar and choose the atoms you want to use: Atoms are the building blocks of molecules or ions. The "Atoms" toolbar in molecular drawing software contains options to select the type of atom you want to use, such as carbon, oxygen, or nitrogen. You can also choose to add charges to atoms by selecting the "Charge" button.
Click and drag the atoms to the canvas and connect them with bonds to form the desired molecule: Once you have selected the atoms and bonds you want to use, you can start drawing your molecule or ion on the canvas. Click on an atom in the "Atoms" toolbar and drag it to the canvas. Connect atoms with bonds by selecting the bond type you want to use and clicking on the atoms you want to connect.
Use the "Advanced Template" toolbar to add functional groups or complex structures to your molecule: The "Advanced Template" toolbar in molecular drawing software allows you to add functional groups or complex structures to your molecule. These can include things like amino acids, nucleotides, or specific ring structures.
Save the molecule as a file or copy and paste it to another application as needed: Once you have finished drawing your molecule or ion, you can save it as a file on your computer or copy and paste it into another application as needed. This can be useful if you want to include your molecule or ion in a report, presentation, or other document.
Learn more about Molecules here:
https://brainly.com/question/19922822
#SPJ4

True or false; A solution always contains only one solvent.
Answers
A solution is defined as a mixture of two or more substances, usually, a solute and a solvent, and the difference between these two are in quantity, solute represents the smallest amount and solvent will represent the highest amount, and while you can have more than one solute, you can only have one solvent for a solution. Therefore the statement is true
Define [Fluid compressibility, Solution-gas/liquid ratio, Fluid FVF, Fluid densities, and Fluid viscosities], write their equations, symbols, units \& correlations. (25-points)
Answers
1. Fluid compressibility (C): Fluid compressibility refers to the measure of how much a fluid's volume changes in response to a change in pressure.
2. Solution-gas/liquid ratio (SGLR): The solution-gas/liquid ratio represents the volume of gas dissolved in a given volume of liquid at a specific pressure and temperature.
3. Fluid formation volume factor (FVF): The fluid formation volume factor represents the ratio of the volume of a fluid at reservoir conditions (pressure and temperature) to its volume at surface conditions.
4. Fluid densities (ρ): Fluid densities refer to the mass per unit volume of a fluid.
5. Fluid viscosities (μ): Fluid viscosities represent the measure of a fluid's resistance to flow.
1. Equation: C = -1/V * dV/dP
Symbol: C
Unit: 1/Pascal (Pa^-1)
Correlation: The compressibility of fluids can vary depending on the fluid type. For ideal gases, the compressibility is inversely proportional to pressure.
2.Equation: SGLR = V_gas / V_liquid
Symbol: SGLR
Unit: Volumetric ratio (e.g., scf/bbl)
Correlation: The solution-gas/liquid ratio is influenced by the pressure and temperature conditions, as well as the composition of the fluid.
3. Equation: FVF = V_reservoir / V_surface
Symbol: FVF
Unit: Volumetric ratio (e.g., bbl/STB)
Correlation: The fluid formation volume factor depends on the composition and properties of the fluid, as well as the reservoir conditions.
4. Equation: ρ = m / V
Symbol: ρ
Unit: Mass per unit volume (e.g., kg/m^3)
Correlation: Fluid densities can vary depending on the type and composition of the fluid. For example, water has a density of approximately 1000 kg/m^3.
5. Equation: No single equation; viscosity is measured experimentally using viscometers.
Symbol: μ
Unit: Pascal-second (Pa·s) or centipoise (cP)
Correlation: The viscosity of a fluid is influenced by temperature and pressure. Different fluids exhibit different viscosities, ranging from low-viscosity fluids like water to high-viscosity fluids like heavy oil.
To know more about Fluid formation volume factor (FVF)
https://brainly.com/question/31458735
#SPJ11
a compound consists of two or more different that are chemically bonded together in fixed proportions. a compound can be broken down into simpler substances, a process that involves a(n) change.
Answers
In chemistry, a compound is formed when two or more chemical elements are chemically bonded. These are the elements in the periodic table that each have their symbol. Every chemical compound always has the same ratio of elements in it, or chemically changes it.
You can separate the elements of a compound and return them to their basic, simplest elemental form. Elements cannot be separated into simpler substances.
This question is a type of fill-in-the-blank:
A compound consists of two or more different ________ that are chemically bonded together in fixed proportions.
A compound can be broken down into simpler substances, a process that involves a(n) ________ change.
Learn more about compounds at https://brainly.com/question/26487468
#SPJ4
Why are small quantities of chlorofluorocarbons so harmful to the ozone layer? a. The chlorofluorocarbons act like ultraviolet radiation causing large amount of ozone to be produced. b. The chlorine from the chlorofluorocarbons reacts with free molecules of oxygen causing a stop in ozone production. c. Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone. d. Chlorofluorocarbons absorb ultraviolet radiation, preventing the formation of ozone.
Answers
Answer:
Why are small quantities of chlorofluorocarbons so harmful to the ozone layer? Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone.
Explanation:
The statement for small quantities of chlorofluorocarbons so harmful to the ozone layer is "Free oxygen atoms can replace the chlorine in chlorine monoxide, releasing a free atom of chlorine which can then recombine with an oxygen atom in ozone, destroying more ozone."
What is ozone layer?The ozone layer is a thin layer of air in the Earth's atmosphere that absorbs nearly all of the sun's damaging UV radiation.
What is CFCs?CFCs (chlorofluorocarbons) are harmless and nonflammable compounds made up of carbon, chlorine, and fluorine atoms.
The earth's protective ozone layer is destroyed by chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), and halons, which shield the earth from damaging ultraviolet (UV-B) rays released by the sun. CFCs and HCFCs also warm the earth's lower atmosphere, causing global climate change.
When some substances are exposed to high UV radiation in the stratosphere, they emit chlorine or bromine. Ozone-depleting chemicals are compounds that contribute to ozone depletion (ODS). Chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform are examples of ODS that produce chlorine. Halons and methyl bromide are two ODS that emit bromine.
Because there isn't much ozone in the atmosphere, what little there is is critical for protecting the Earth's surface from excessive UV light from the Sun. It turns out that it reacts with chlorine, which means that chlorine effectively eliminates ozone.
When the chlorine in CFCs combines with ultraviolet light, it releases chlorine, which then reacts with ozone, reducing the protection humans get from ultraviolet light, allowing more CFCs to release chlorine, and so on. Multiple ozone molecules will interact with one free chlorine atom, which is free because UV light has hit the CFC molecule. As a result, the damage it can cause is likely to be significantly more than you might imagine.
Hence the correct option is c.
Learn more about ozone layer and CFCs here
https://brainly.com/question/14330630
#SPJ2
answer this step by step pls
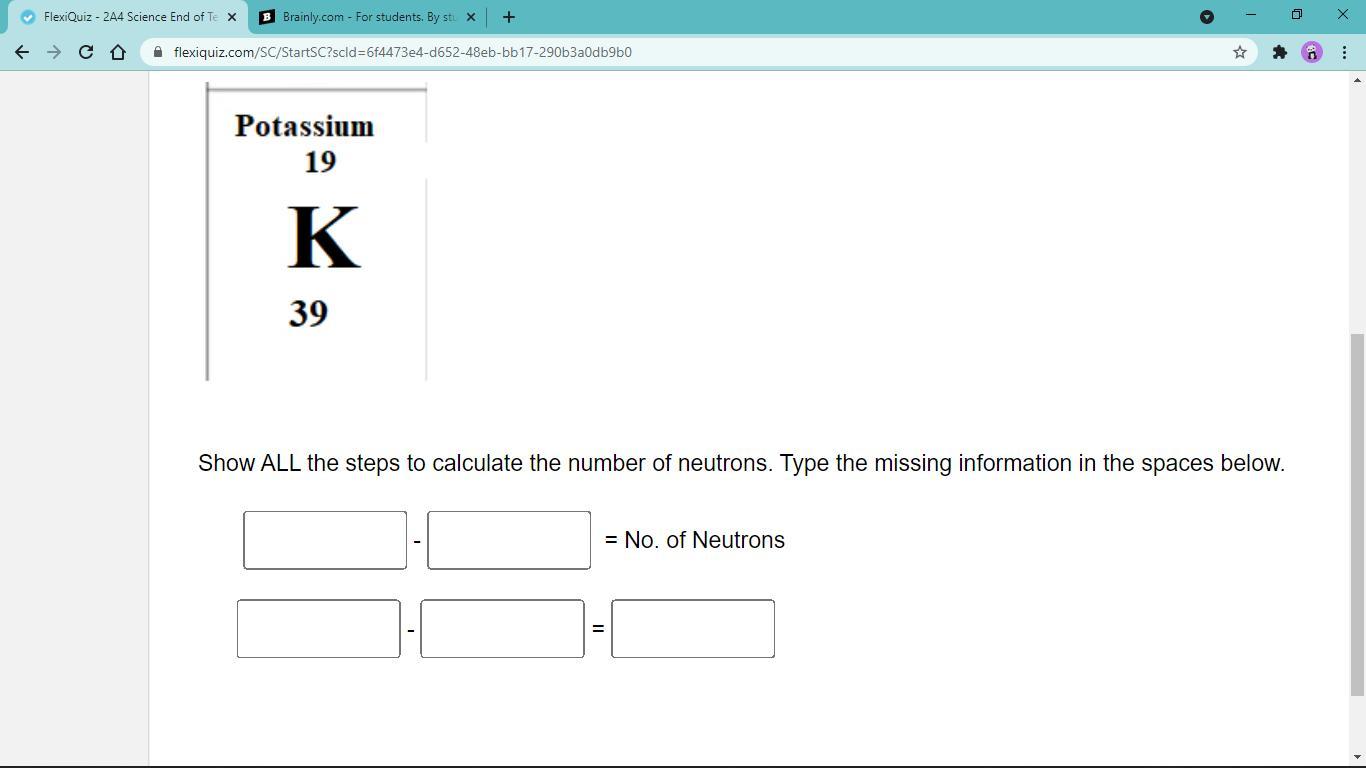
Answers
Answer:
neutrons = mass number - atomic number
. = 39-19 =20
Does changing the number of neutrons change the identity of the element you have built ?
Answers
Answer:
If you change the number of neutrons somehow, nothing will happen because it carry's no charge at all.
Explanation:
Which of the following is a producer? O mouse cactus O snake O goose
Answers
Answer:
cactus
Explanation:
Its a plant so it makes it own food
How many grams are there in 7.5E10 atoms of tungsten (W)?
Answers
Answer:
1 mole is equal to 1 moles Tungsten, or 183.84 grams. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between moles Tungsten and gram.
Explanation:
Salt from evaporated seawater has been formed from
O a. artifical means
O b. solution
O c. volcanoes
O d. magma
Answers
Answer:
a
Explanation:
its formed from sodium and sodium metal is produced by electrolysis of dry molten sodium chloride.
A car is moving with the velocity of 20m/s. After 5 seconds it's velocity becomes 50m/s. Find its acceleration.
Answers
Answer:
6 m/s^2
Explanation:
a= (v2 - v1)/t
= (50-20)/5
=6
Answer:
6 m/s²
Explanation:
Given, Initial velocity (u) = 20m/s
Final velocity (v) = 50m/s
time taken (t) = 5 sec
Now,
Acceleration = v-u/t
= 50-20/5
= 30/5
= 6 m/s²
what is the bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals?
Answers
The bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Bond order is defined as the number of electrons in bonding molecular orbitals minus the number of electrons in antibonding molecular orbitals divided by two. As a result, we may determine the bond order of this diatomic particle by the formula: Bond order = (number of bonding electrons - number of antibonding electrons) / 2
Bond order = (8 - 5) / 2
Bond order = 1.5.
This diatomic molecule, according to the bond order, is a stable molecule since the bond order is greater than 1, indicating that it is a double bond. The molecule has an overall bond strength that is greater than a single bond, but not as strong as a triple bond. So therefore he bond order for a second-period diatomic particle containing five electrons in antibonding molecular orbitals and eight electrons in bonding molecular orbitals is 1.5
Learn more about bond order at:
https://brainly.com/question/30641030
#SPJ11
✓ What kind of carbon storages would be the most affected by humans?
Answers
Answer: Burning fuels?
Explanation: I’m not very sure actually
Drag each tile to the correct image. Match each hydrocarbon class to its structure. carboxylic acid amine halocarbon alcohol

Answers
Answer:
1. Amine.
2. Alcohol.
3. Carboxylic Acid.
4. Halocarbon.
Explanation:

The correct answer according to the tile are Amine, Alcohol, Carboxylic acid, Halocarbon.
How can hydrocarbons be classified based on their structure?
Hydrocarbons can be classified as either aromatic or aliphatic compounds, depending on the presence of a benzene ring.
What is the most common classification of hydrocarbons?
Alkanes are hydrocarbons in which all of the bonds are single bonds. Alkenes are hydrocarbons that contain a carbon-carbon double bond.
Learn more about hydrocarbon here : brainly.com/question/490217
#SPJ2
lipids are hydrophilic, which means they do not dissolve in water. true or false
Answers
False. Lipids are hydrophobic, which means they do not dissolve in water. This is because lipids are non-polar molecules and water is a polar solvent.
However, lipids can dissolve in other non-polar solvents such as oils and fats.
False. Lipids are actually hydrophobic, meaning they do not dissolve in water. Hydrophilic substances, on the other hand, do dissolve in water.
to know more about Lipids intake pls visit:
https://brainly.com/question/29148559
#SPJ11
plssss help i am giving out a lot of points

Answers
Answer question number 14. The question is in the image.


Answers
We have an alkene due to the presence of a double bond in the chain. Alkenes are named by placing the ending -ene at the end of the word.
Now, to name the initial part we must count the carbons present in the molecule. We start by counting from the side closest to the double bond so that the carbon that has the double bond has the lowest possible value. Let's see how the numbering would be:
We see that the molecule has 5 carbons, so it will start with pent-, and the double bond is located at carbon number two. So the name of the molecule will be: 2-pentene
Answer: (1) 2-pentene

How many hydrogen atoms are present in 2-methyl-2-butene?
Answers
There are three kinds of Hydrogen in 2-methyl-2-butene
The correct question is
How many kinds of hydrogen atoms are present in 2-methyl-2-butene
What is an atom ?Atom was discovered by Dalton's Atomic theory , he predicted that each element consist of large number of a very small thing known as atom.
In the structure of 2-methyl-2-butene there are 3 types oh Hydrogen
1. Attached to the Carbon atom connecting the methyl ion to the butene
2. Connected to the last Carbon atom
3. Connected to the Carbon bond having double bond.
Therefore there are three kinds of Hydrogen in 2-methyl-2-butene.
To know more about Atom
https://brainly.com/question/1566330
#SPJ1
PLEASE HELP MEEEE!!!! 10 POINTS + BRAINLYEST!!!!!!
NO LINKS PLEASE!!!!!
From a Christian perspective, the beauty of chemical elements reflects all but which of the following?
God’s glory
God's judgement
God’s power
God’s wisdom
Answers
Answer:
God's power is not reflected here.
Hey everyone! Trying to figure out the name for this question. If you could help me, that would be amazing!
