Atomic size increases down a main group and decreases across a period. in a transition series, size remains relatively constant.
a. true
b. false
Answers
The given statement "Atomic size increases down a main group and decreases across a period. In a transition series, size remains relatively constant" is false because atomic size increases down a main group but it is not constant as there are fluctuations in between.
Atomic size increases down a main group because there are more occupied energy levels or shells, which increases the distance between the nucleus and the valence electrons.
Atomic size decreases across a period because the number of valence electrons increases while the number of occupied energy levels remains the same. This causes an increase in effective nuclear charge, which pulls the valence electrons closer to the nucleus and reduces the atomic radius.
In a transition series, atomic size generally decreases across the series. However, it is not constant as there are some fluctuations depending on the electronic configuration of the elements.
To learn more about periodic properties refer: https://brainly.com/question/13837536
#SPJ11
Related Questions
an atom of chlorine has an amount number of 17 and a mass number of 37. how many protons neutrons, and electrons are in the atom?
Answers
Answer: There are 20 protons neutrons in the atom
I am a celestial body that does not produce light. I orbit a planet
Answers
What types of forces allow an object to stay at rest or at a constant velocity?
a. Balanced
b. Unbalanced
C. Accelerated
d. Applied
Answers
Explanation: It is NOT possible for just three forces to be acting upon an object and they still balance each other. A free-falling object experiences a balance of forces. Balanced forces cause stationary objects to remain at rest and moving objects to come to rest. Unbalanced forces cause objects to move.
Picture for reference:

Help me please
Thank you
Have a great day :)

Answers
The balanced chemical equation of the combustion reaction of the given gasoline, octane can be written as follows:
\(\rm C_{8}H_{18} + \frac{25}{2} O_{2} \rightarrow 8CO_{2} + 9H_{2}O\)
What is combustion ?Combustion is type of reaction in which gases reacts with atmospheric oxygen to give carbon dioxide and water. Hydrocarbon gases easily undergo combustion and they are used as fuels.
Octane undergo combustion to produce 8 moles of carbon dioxide and 9 moles of water. The number of moles of oxygen gas in this reaction is 25.
Therefore, the coefficients of the reaction are in the order 1, 25/2, 8 and 9. The reaction can be balanced using simple integers to multiple both side based to equate each reactants equal in number on both side.
Find more on combustion :
https://brainly.com/question/13153771
#SPJ1
Which form of energy is directly related to the measure of the average kinetic energy of the particles in a substance?
thermal energy
potential energy
mechanical energy
electromagnetic energy
Answers
Answer:
thermal energy
Explanation:
PLS PLS PLS PLS HELP IM STRUGGLING
Which of the tests above would provide the most useful information to determine whether the sample is a pure substance or a mixture?
A. 4 only
B. 1 and 4
C. 1,2, and 3
D. 1 only

Answers
Answer:
A. 4 only
Explanation:
One of the tests for purity is by determining the melting point of a substance. A pure substance has a sharp meting point.
If the solid in question is a pure solid, when it is heated, it will exhibit a sharp melting point. Mixtures however melt over a range of temperatures due to the presence of impurities in the solid.
Delta H in chemistry means????
Answers
Answer:
In chemistry, the letter "H" represents the enthalpy of a system
Explanation:
What human activities contribute to global warming
Answers
Answer:
Human climate drivers include heat-trapping emissions from burning coal, gas and oil in power plants and cars; cutting down and burning forests; tiny pollution particles (aerosols); black carbon pollution more commonly referred to as soot; and changes in land use that also affects Earth's albedo.
Explanation:
The burning of fossil fuels or even driving a car can lead to global warming. This is due to the greenhouse gases released in the air from these activities. This is even primarily why electric cars were invented. Deforestation can also lead to global warming due to less plants absorbing carbon dioxide.
suppose that at the end of reaction 1 the level of the aqueous solution were 26 cm higher inside the buret than outside. compared to ambient pressure, the pressure of the gas inside the buret would be:
Answers
Answer:
The pressure of the gas inside the buret would be higher than ambient pressure if the level of the aqueous solution were 26 cm higher inside the buret than outside.
This is because the gas inside the buret is being compressed by the weight of the liquid column above it. The higher the liquid column, the greater the pressure on the gas. So when the level of the aqueous solution is 26 cm higher inside the buret than outside, it means that there is a greater weight of liquid pushing down on the gas, which increases the pressure of the gas.
It's important to note that the pressure inside the buret is not only due to the liquid column but also due to the atmospheric pressure. So the pressure of the gas inside the buret will be higher than ambient pressure but not only by 26cm of liquid column.
What will be the next step in this calculation?
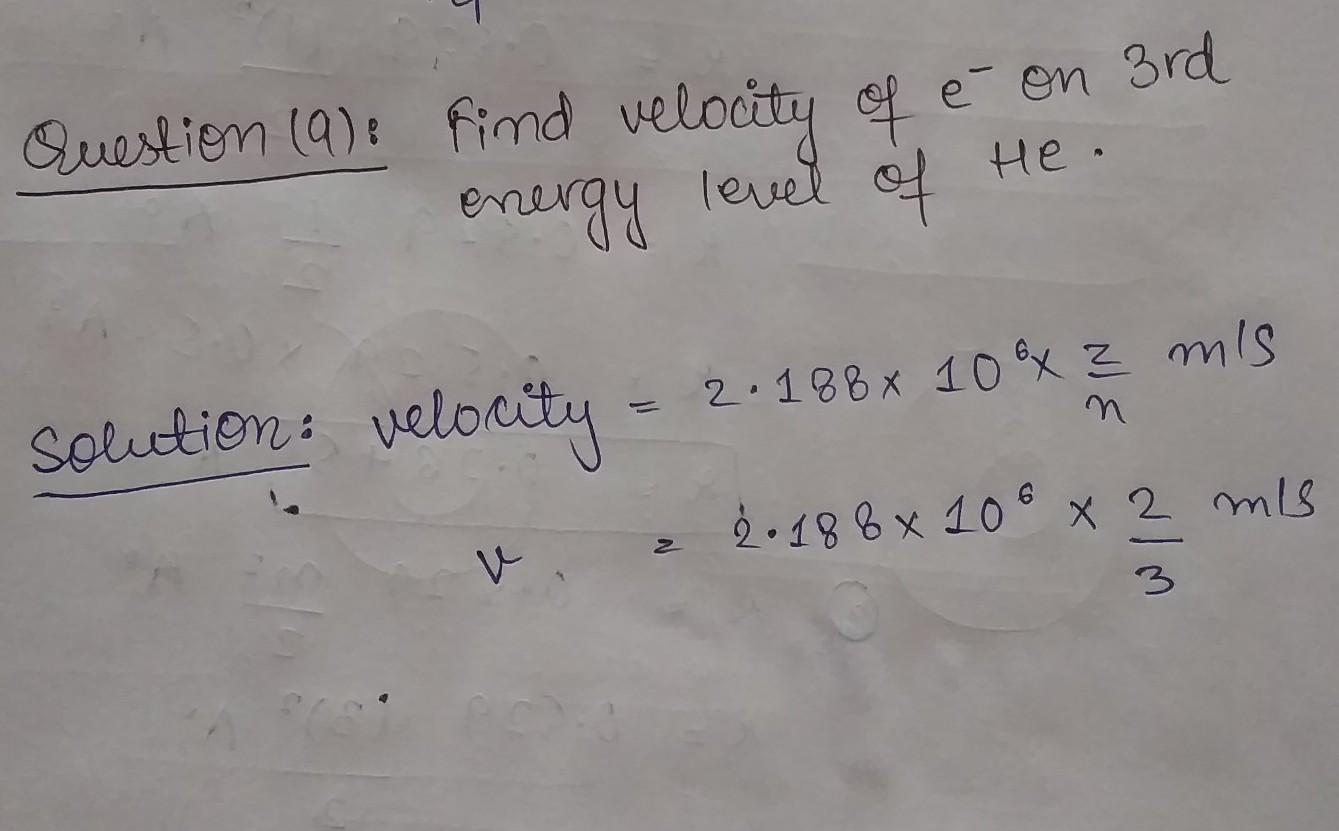
Answers
V = 2.188x10^6 x 2
————————
3
V = Answer
————
3
V = (what you got)
Try finding them with calculator or something.
Hope this helps.Good luck ✅.And I wish this can still help.if the illustration of thomson's atom represents a neutral atom, what must be true about the total amount of positive charge and the total amount of negative charge?
Answers
The illustration of Thomson's atom represents a neutral atom. In this case, the total amount of positive charge and the total amount of negative charge must be equal. This means that there are equal numbers of protons and electrons in the atom. This is what makes the atom neutral.
What is a neutral atom?A neutral atom is an atom that has no electrical charge. An atom is neutral because it has the same amount of positively charged protons and negatively charged electrons. The nucleus of an atom contains protons, which are positively charged particles. Electrons, which are negatively charged particles, are located in the atom's electron cloud around the nucleus.
Electrons, protons, and neutrons are the three components of atoms. Electrons are negatively charged, protons are positively charged, and neutrons have no charge. Electrons are found outside the nucleus of the atom and are continually moving at high speeds.
In summary, if the illustration of Thomson's atom represents a neutral atom, then the total amount of positive charge and the total amount of negative charge must be equal. This means that there are equal numbers of protons and electrons in the atom. This is what makes the atom neutral.
Learn more about Thomson's atom on the given link:
https://brainly.com/question/1597441
#SPJ11
The average atomic mass of zinc is 65.37 amu, and it's atomic number is
30. How many ELECTRONS does zinc have?
Answers
Answer:
30
Explanation:
The number of protons determines an element's atomic number and is used to distinguish one element from another. ... Together, the number of protons and the number of neutrons determine an element's mass number.
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
An aqueous solution contains
32.7% KCI (weight/weight %).
What is the mole fraction of KCI in
this aqueous solution?
Molar Mass
KCI: 74.55 g/mol
H₂O: 18.016 g/mol
Enter text here
Enter
Answers
Answer:
0.105 KCl
Explanation:
(Step 1)
If the solution is 32.7% KCl, we can assume that there are 32.7 grams KCl in the solution. This means that there would be 67.3 grams H₂O (100% - 32.7% = 67.3%) in the solution.
32.7% KCl = 32.7 g KCl
67.3% H₂O = 67.3 g H₂O
(Step 2)
Now, we need to convert both the solute (KCl) and the solvent (H₂O) into moles. This can be done by multiply the grams of each substance by their molar masses.
32.7 g KCl 1 mole
------------------ x -------------------- = 0.439 mole KCl
74.55 g
67.3 g H₂O 1 mole
------------------ x ----------------- = 3.74 moles H₂O
18.016 g
(Step 3)
Finally, we can calculate the mole fraction of KCl using the following equation. The final answer should have 3 sig figs to match the given value with the lowest amount of sig figs (32.7 g).
moles of solute
Mole Fraction = -----------------------------------------------------
moles of solute + moles of solvent
0.439 mole KCl
Mole Fraction = ------------------------------------------------------
0.439 mole KCl + 3.74 moles H₂O
Mole Fraction = 0.105 KCl
how do i set up an electron configuration

Answers
Answer:
Electron configuration can be carried out in two steps which can be:
Sublevel notation
Shell notations.
In the sublevel notation, the sequence of filling electrons into the orbitals of the sublevels are guided by some principles:
the maximum number of electrons in the orbital of sublevels are two for s-sublevel, six for p-sublevel, ten for d-sublevel and fourteen for f-sublevel. This indicates that the maximum number of electrons in an orbital is two.
Aufbau's prinicple states that sublevel with lower energies are filled up before those with higher energies.
Pauli exclusion principle states that no two electrons in an atom can have the same set of the four quantum numbers.
Hund's rule states that electrons go into degenerate orbitals singly first before pairing occurs.
Using the shell notation, numbers are used to denote the sum of electrons in all orbitals each energy level.
For an atom of Be:
Sublevel notation for the 4 electrons 1s²2s²
Shell notation 2,2
Other representation is using the electron dot structure.
state two advantages of the environmental method over the chemical method of controlling mosquito
Answers
The mosquitoes play an important role which serve as pollinators and as a food source for other wildlife. It is often said that the mosquitoes serve no purpose other than to annoy humans.
What are the environmental methods to control mosquitoes?The mosquito breeding can be eliminated at home by removing unused plastic substances, old tires, buckets and also by eliminating stagnant water and polluted water.
In order to reduce the population of mosquito naturally, clean our surroundings and home clean, burn the wastes, plastics, etc. Dumping or removing stand water in and around our home is another way to control larvae.
To know more about mosquito, visit;
https://brainly.com/question/7690716
#SPJ1
explain why the first ionization energy is much lower than the second ionization energy for an atom of sodium.
Answers
The lower first ionization energy of sodium is due to the relatively weak attraction between the outermost electron and the nucleus, as well as the shielding effect provided by the inner electrons.
The ionization energy refers to the amount of energy required to remove an electron from an atom or ion in its gaseous state. In the case of sodium, the first ionization energy is significantly lower than the second ionization energy. This can be explained by understanding the electron configuration and the principles of electron shielding and effective nuclear charge.
Sodium has an atomic number of 11, meaning it has 11 protons in its nucleus and 11 electrons surrounding it. These electrons are arranged in energy levels or shells, with the first shell containing 2 electrons and the second shell containing 8 electrons. The outermost electron in sodium is in the third energy level.
The first ionization energy is the energy required to remove the outermost electron from the atom. In sodium, this electron is relatively far from the nucleus and experiences less attraction to the positively charged protons.
Additionally, the outer electron in sodium experiences significant electron shielding from the inner electrons, meaning that the inner electrons partially shield the outer electron from the full attractive force of the nucleus.
As a result, it is easier to remove the outermost electron in sodium, and hence, the first ionization energy is relatively low. Once the outermost electron is removed, sodium becomes a positively charged ion (Na+).
The second ionization energy refers to the energy required to remove an electron from the Na+ ion, which now has a stronger effective nuclear charge due to the reduced electron-electron repulsion and decreased shielding effect. Consequently, it is more difficult to remove an electron from the Na+ ion, leading to a higher second ionization energy compared to the first ionization energy.
For more such questions on ionization energy visit:
https://brainly.com/question/30831422
#SPJ8
Is using matches to light a candle physical or chemical change ?
Answers
Answer:
It's a chemical change.
Explanation:
The process of burning (as opposed to evaporating) is a chemical reaction, a chemical change. The wax molecules are undergoing a chemical change; they are changing into different molecules by reacting with a substance in the air.
Pls Answer this giveing brainilest an 20 points

Answers
Answer:
Deserts and oceans appear to be far from one another will little in common, but in fact, they're connected in an intresting way.Water from the open evaporates into clouds that travel and eventually empty the water onto the land,creating much-needed sources of water in dessert areas.Meanwhile,winds from the dessert sweep up billions of tons of dust intosky,at least a quarter of which falls into the ocean and provides nutrients for marine life.
Explanation:
Which of these is not a mixture?
A) Salt
B) Cooking oil
C) Tea leaves
D) Milk
ty
Answers
Answer:
the answer is salt because it has a uniform and definite composition
Explanation:
sorry for being inactive (if you know me) i have face-to-face school and barely any homework so thats my excuse haha.
Answers
Answer:
relatable a.f
Explanation:
Answer:
Thank you for free point :)
Explanation:
A voltaic cell consists of Cr/Cr³⁺ and Cd/Cd²⁺ half-cells with all components in their standard states. After 10 minutes of operation, a thin coating of cadmium metal has plated out on the cathode. Describe what will happen if you attach the negative terminal of a dry cell (1.5 V) to the cell cathode and the positive terminal to the cell anode.
Answers
In a voltaic cell, if we attach the negative terminal of a dry cell (1.5 V) to the cell cathode and the positive terminal to the cell anode, there will be oxidation of the Cd and reduction of Cr
The oxidation takes place in the Cd electrode, which is the anode, and it gets oxidized to Cd²⁺ (aq). Hence, the Cd electrode loses mass as the cell reaction moves forward.
A voltaic cell is an electrochemical cell that uses a chemical response to produce electric energy. The crucial components of a voltaic mobile: The anode is an electrode where oxidation occurs. The cathode is an electrode where a discount occurs.A galvanic cell or voltaic cellular, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cellular wherein an electric-powered modern is generated from spontaneous Oxidation-reduction reactions
Learn more about voltaic cells here:-https://brainly.com/question/3930479
#SPJ4
A 2.50 mL aliquot of a 0.10 M HCl solution is diluted to a final volume of 25.00 mL. What is the molarity of this first dilution solution?Then a second dilution was made by taking 8.00 mL of the first dilution and diluting it to 50.00 mL. What is the molarity of this second dilution?
Answers
The molarity of the first dilution solution is 0.010 M while the molarity of the second dilution is 0.0016 M.
To find the molarity of the first dilution solution, we can use the formula:
M₁V₁ = M₂V₂
where M₁ is the initial molarity, V₁ is the initial volume, M₂ is the final molarity, and V₂ is the final volume. Plugging in the values given:
M₁ = 0.10 M
V₁ = 2.50 mL = 0.00250 L
M₂ = ?
V₂ = 25.00 mL = 0.02500 L
0.10 M x 0.00250 L = M₂ x 0.02500 L
M₂ = 0.010 M
To find the molarity of the second dilution, we can use the same formula:
M₁V₁ = M₂V₂
but now we are using the first dilution as our initial solution. Plugging in the values given:
M₁ = 0.010 M
V₁ = 8.00 mL = 0.00800 L
M₂ = ?
V₂ = 50.00 mL = 0.05000 L
0.010 M x 0.00800 L = M₂ x 0.05000 L
M₂ = 0.0016 M
Learn more about dilution here: https://brainly.com/question/27097060
#SPJ11
The table below contains characteristics of four different elements. Characteristics of Four Elements Element Number of Protons Number of Valence Electrons 1 ca 4 2. 11 1 1 3 18 8 4 37 1 1 What conclusion can be made from the information above? Element 3 is a metal and Element 1 is a metalloid. Element 2 is the same element as Element 3. Element 1 has very similar chemical properties as Element 4. Element 2 has very similar chemical properties as Element 4.

Answers
Answer:
The correct option is "Element 2 has very similar chemical properties as Element 4"
Explanation:
Both element 2 and element 4 have the same number of valence electrons; meaning they will be found in the same group (group 1) of the periodic table. Elements in the same group of the periodic table exhibit similar chemical properties. For confirmation, element 2 is sodium (based on the atomic number of 11 provided) while element 4 is rubidium (based on the atomic number of 37 provided) - the two elements are found in group 1 of the periodic table.
NOTE: Number of protons is the same as atomic number
If you start with 3 moles of sodium and 3 moles of chlorine to produce sodium chloride, what is the limiting reagent?(you will need to balance the equation first.) na cl2 -> nacl
Answers
Sodium(Na) is the limiting reagent.
What is Limiting reagent?The reactant that is totally consumed during a reaction, or the limiting reagent, decides when the process comes to an end. The precise quantity of reactant required to react with another element may be estimated from the reaction stoichiometry.
How do you identify a limiting reagent?
The limiting reactant is the one that is consumed first and sets a limit on the quantity of product(s) that can be produced. Calculate how many moles of each reactant are present and contrast this ratio with the mole ratio of the reactants in the balanced chemical equation to get the limiting reactant.
Start by writing the balanced chemical equation that describes this reaction
\(2Na_{(s)} + Cl_{2 (g)} -- > 2NaCl_{(s)}\)
Notice that the reaction consumes 2 moles of sodium metal for every 1 mole of chlorine gas that takes part in the reaction and produces 2 moles of sodium chloride.
now we can see that we have 3 moles of sodium and 3 moles of chlorine, according to question. so, we can say that sodium is the limiting reagent in the given situation.
to learn more about Limiting Reagent go to - https://brainly.com/question/14222359
#SPJ4
The answer to this question

Answers
Answer:
C/the third one
Explanation:
it’s correct
Answer:
D. 24.98
Explanation:
23.985+24.9858+25.9826=74.9534÷3=24.9844666667
rounded to nearest hundredth=24.98
indicate whether each of the following is characteristic of the fission or fusion process, or both: a. very high temperatures are required to initiate the reaction. b. less radioactive waste is produced. c. hydrogen nuclei are the reactants. d. large amounts of energy are released when the nuclear reaction occurs.
Answers
Let's analyze each characteristic in relation to the processes of fission and fusion:
a. Very high temperatures are required to initiate the reaction.
Fission: High temperatures are not required to initiate the fission process. Fission occurs when a heavy nucleus is split into two smaller nuclei1.Fusion: Very high temperatures are required to initiate the fusion process. Fusion occurs when two light nuclei combine to form a heavier nucleus. These high temperatures are necessary to overcome the electrostatic repulsion between the positively charged nuclei.b. Less radioactive waste is produced.
Fission: Fission reactions can produce a significant amount of radioactive waste due to the splitting of heavy nuclei and the formation of radioactive isotopes.Fusion: Fusion reactions generally produce less radioactive waste compared to fission reactions. The fusion process involves lighter nuclei and does not produce as many long-lived radioactive isotopes.c. Hydrogen nuclei are the reactants.
Fission: Hydrogen nuclei are not typically the reactants in the fission process. Fission reactions involve heavy nuclei such as uranium or plutonium.Fusion: Hydrogen nuclei, specifically isotopes such as deuterium and tritium, are the primary reactants in the fusion process.d. Large amounts of energy are released when the nuclear reaction occurs.
Fission: Fission reactions can release large amounts of energy. This occurs when the binding energy per nucleon of the resulting nuclei is greater than that of the initial nucleus.Fusion: Fusion reactions also release large amounts of energy. The energy is released when the binding energy per nucleon of the resulting nucleus is greater than that of the reactant nuclei.Summarizing the characteristics for fission and fusion:
Fission:
Very high temperatures are not required to initiate the reaction.More radioactive waste is produced.Hydrogen nuclei are not the reactants.Large amounts of energy are released.Fusion:
Very high temperatures are required to initiate the reaction.Less radioactive waste is produced.Hydrogen nuclei are the reactants.Large amounts of energy are released.Based on these characteristics, we can conclude that:
a. Very high temperatures are characteristic of fusion.
b. Less radioactive waste being produced is characteristic of fusion.
c. Hydrogen nuclei as reactants are characteristic of fusion.
d. Large amounts of energy being released are characteristic of both fission and fusion.
To know more about fission and fusion refer here
https://brainly.com/question/13052582#
#SPJ11
How many moles of Nitrous oxide in 40.0 g of N₂O?
Answers
Answer:
0.909 mole
Explanation:
Given N = 14, O = 16
=> so N20 = 14(2) + 16 = 44 g/mole
so number of mole of 40.0g = (40.0g)/(44 g/mole) = 0.909 mole
Determine the products of the reaction between tin(ii) oxalate and lithium chloride
Answers
The reaction between tin (II) oxalate and lithium chloride is that it forms tin (II) chloride and lithium oxalate, which are the products of the reaction. The balanced chemical equation for the reaction is SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4.
Tin (II) oxalate reacts with lithium chloride to form a precipitate of tin (II) chloride and lithium oxalate. The reaction between tin (II) oxalate and lithium chloride is given below.
SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4
The balanced chemical equation for the reaction is as follows:
SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4 .
SnC₂O₄ is tin (II) oxalate, while LiCl is lithium chloride.
SnCl₂ is tin (II) chloride, while Li₂C₂O4 is lithium oxalate.The products of the reaction between tin (II) oxalate and lithium chloride are tin (II) chloride and lithium oxalate. Tin (II) chloride is a white crystalline powder that is soluble in water, whereas lithium oxalate is a white solid that is insoluble in water.The reaction between tin (II) oxalate and lithium chloride is a double displacement reaction, which is also known as a metathesis reaction. When a double displacement reaction takes place, two compounds exchange their cations and anions, resulting in the formation of two new compounds.
The reaction is a double displacement reaction or metathesis reaction where two compounds exchange their cations and anions to form two new compounds.
To know more about double displacement reaction visit:
brainly.com/question/29740109
#SPJ11
tiana is a chemist who is making a chemical to add to swimming pools
Answers
Tiana is developing a chemical additive to be used in swimming pools.
What is chemical additive?Chemical additives are substances added to products to alter or improve their performance. They are used in a wide range of consumer products and industrial processes for a variety of purposes including improving shelf-life, enhancing flavor, or increasing the efficiency of a process. Common examples of chemical additives are preservatives, colorants, emulsifiers, antioxidants, stabilizers, and thickeners.
This additive is designed to help keep the pool clean and sanitary, by removing bacteria and other contaminants from the water. The additive is also designed to help balance the pH of the pool water, to ensure that it is safe for swimming and does not irritate swimmers' skin or eyes. The chemical additive must also be safe to use, and must not cause any adverse reactions in swimmers. Tiana's work involves testing different chemical compounds to find the most effective and safe additive for pool water.
To learn more about bacteria
https://brainly.com/question/30307005
#SPJ1
What is the predicted shape, bond angle, and hybridization for CH3? A) trigonal planar, 120°, sp2 B) trigonal planar, 120°, sp3 C) trigonal planar, 109.5°, sp2 D) trigonal pyramidal, 120°, sp2 E) trigonal pyramidal, 109.5°, sp2
Answers
However, assuming that CH3 is a part of a larger molecule, we can predict its shape, bond angle, and hybridization based on the bonding theory.
Since CH3 has three groups of valence electrons surrounding the central carbon atom, we can predict its shape to be trigonal planar. The bond angle between each of the three hydrogen atoms and the central carbon atom is predicted to be 120°. To determine the hybridization of the carbon atom, we can count the total number of electron groups (3 bonding groups + 0 lone pairs = 3 electron groups).
Based on this, we can predict the hybridization of the carbon atom to be sp2, where the s orbital and two of the p orbitals of the carbon atom hybridize to form three equivalent sp2 orbitals that are oriented in a trigonal planar arrangement. Therefore, the answer would be option A) trigonal planar, 120°, sp2.
To know more about hybridization visit:
https://brainly.com/question/14358519
#SPJ11