at the end of glycolysis, in what molecule(s) can one not find the energy that was contained in the chemical bonds of glucose? select all that apply.
Answers
Two molecules of ATP, two molecules of NADH, and two molecules of pyruvate are net products of glycolysis. Pyruvate is where the majority of the energy is kept. It will travel through the Krebs cycle to continue to oxidize.
Which of the following does not result from glycolysis to produce ATP?
Carbon dioxide is the appropriate response to this query. Glycolysis does not result in the production of carbon dioxide.
What results from glycolysis in the absence of oxygen?
Two molecules of pyruvate, adenosine triphosphate (ATP), nicotinamide adenine dinucleotide (NADH), and water are the products of glycolysis. Both aerobic and anaerobic species engage in glycolysis. Pyruvate is fermented into lactic acid, ethanol, and CO2 when oxygen is not present
To know more about glycolysis visit;
https://brainly.com/question/27178607
#SPJ4
Related Questions
the salt bridge contains a solution of a strong electrolyte. group of answer choices true false flag question: question 3 question 32 pts the reducing agent is the substance being oxidized in the reaction. group of answer choices true false
Answers
The given statement "the salt bridge contains a solution of a strong electrolyte" is true because a salt bridge contains a strong electrolyte solution to maintain electrical neutrality in an electrochemical cell . For the second question, the given statement "the reducing agent is the substance being oxidized in the reaction" is false because the reducing agent is the substance being oxidized, as it donates electrons to reduce another substance in the redox reaction .
The solution in the salt bridge typically consists of an electrolyte, which is a substance that dissociates into ions when dissolved in water. Strong electrolytes are substances that dissociate completely into ions in solution, such as sodium chloride (NaCl). This ensures that the salt bridge can effectively facilitate the flow of ions between the half-cells.
In a redox reaction, which involves the transfer of electrons between species, the reducing agent is the substance that donates electrons to another species, while the oxidizing agent is the substance that accepts electrons.
Therefore, the substance being oxidized is the oxidizing agent, not the reducing agent. For example, in the reaction 2Mg + \(O_{2}\) → 2MgO, magnesium is the reducing agent because it donates electrons to oxygen, which is the oxidizing agent that accepts electrons. Magnesium is therefore being oxidized, not reduced.
Know more about salt bridge here:
https://brainly.com/question/31196857
#SPJ11
what reaction type is the followong reaction
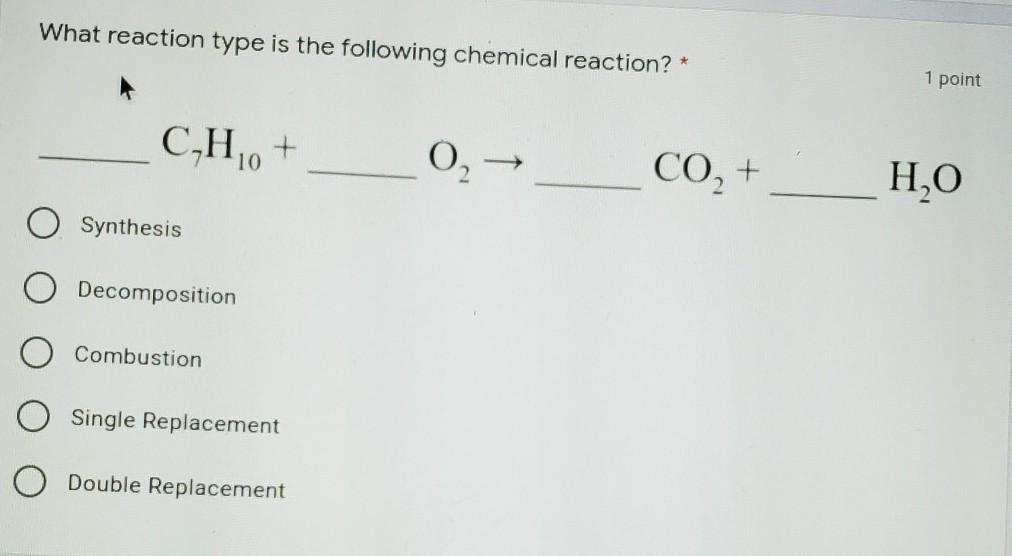
Answers
Answer:
its the fourth one please mark as brainlest
Explanation:
Calculate the mass of 1.25 mol ammonium sulfide, (NH4)2S. *
Answers
Answer:
85g
Explanation:
To convert the moles of a substance to grams we need to know the molar mass of the substance. We, as first, must obtain the molar mass of (NH₄)₂S as follows:
There are 2 atoms of N, 8 of H and 1 of S:
N = 2*14g/mol = 28g/mol
H = 8*1g/mol = 8g/mol
S = 1*32g/mol = 32g/mol
Molar mass: 68g/mol
That means 1 mole of (NH₄)₂S has a mass of 68g.
1.25moles have a mass of:
1.25moles * (68g/mol) =
85gWhich equation below is an example of a single-replacement reaction?
A.6CO2(g) + 6H2O(l) → C6H12O6(aq) + 6O2(g)
B.H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
C.Ca(OH)2(s) Δ→ CaO(s)+ H2O(l)
D.Zn(s) + 2HCl(aq)→ ZnCl2(aq) + H2(g)
Answers
The equation that depicts a single replacement reaction would be: Zn(s) + 2HCl(aq)→ ZnCl2(aq) + H2(g)
Single replacement reactionsThey are also known as single displacement reactions.
They are reactions in which one element takes the place of another in a compound. That is, one element replaces another in a compound.
Looking at all the reactions from A - D, one can see that the only reaction that exemplifies a single replacement reaction is D.
Here, Zn replaced H in HCl.
More on single replacement reactions can be found here: https://brainly.com/question/13328989
[b] Potassium-40 has a half-life of 1.25 billion years. If a rock sample contains W Potassium-40 atoms for every 1000 its daughter atoms, then how old is this rock sample? Your answer should be significant to three digits. Remember to show all your calculations,
Answers
The rock sample is approximately 1.992 billion years old.
Potassium-40 (K-40) has a half-life of 1.25 billion years, which means that after 1.25 billion years, half of the original K-40 atoms would have decayed into daughter atoms. In this particular rock sample, we are given that there are W Potassium-40 atoms for every 1000 daughter atoms.
To determine the age of the rock sample, we need to find the value of W. Since the half-life of K-40 is 1.25 billion years, after each half-life, the ratio of K-40 to daughter atoms will be halved. So, after one half-life, the ratio would be 1:2000 (W:1000).
To calculate the number of half-lives, we can use the equation:
(number of half-lives) = (log(W/1000)) / (log(1/2))
Since we are given W Potassium-40 atoms for every 1000 daughter atoms, we can substitute the ratio into the equation:
(number of half-lives) = (log(W/1000)) / (log(1/2))
(number of half-lives) = (log(W/1000)) / (-0.301)
Simplifying the equation, we find:
(number of half-lives) = -3.32 * log(W/1000)
Since we want to find the age of the rock sample, we multiply the number of half-lives by the half-life of K-40:
Age = (number of half-lives) * (half-life of K-40)
Age = -3.32 * log(W/1000) * 1.25 billion years
By substituting the given value of W and performing the calculations, we can determine the age of the rock sample to be approximately 1.992 billion years.
Learn more about Rock
brainly.com/question/29898401
#SPJ11
with the above information and your knowledge of alkenes, select the statements that are TRUE. 1-Butene is the most stable compound. 2-Methyl-i-propene is the most stable compound. 1-Butene is the least stable compund. 2-Methyl-i-propene is the least stable compound. The more carbon atoms attached to the double bond, the more stable the alkene, A trans isomer is less stable than a cis isomer due to more steric hindrance. sp2 hybridized carbon atoms are more electronegative than sp^3? hybridized atoms.
Answers
The 2-Methyl-1-propene is the most stable compound This is true because it has more substituents (alkyl groups) attached to the double bond, which increases its stability due to the electron-donating effect of the alkyl groups. This makes sp2 hybridized carbon atoms more electronegative.
The 1-Butene is the least stable compound This is true because it has fewer substituents attached to the double bond compared to 2-Methyl-1-propene, making it less stable. The more carbon atoms attached to the double bond, the more stable the alkene This statement is true as well. Alkenes with more carbon atoms attached to the double bond have increased stability due to the electron-donating effect of the alkyl groups. A trans isomer is less stable than a cis isomer due to more steric hindrance: This statement is false. In general, trans isomers are more stable than cis isomers because they have fewer steric hindrances and lower energy conformations. sp2 hybridized carbon atoms are more electronegative than sp3 hybridized atoms: This statement is true. sp2 hybridized carbon atoms have a greater proportion of "s" character (33% s-character and 67% p-character) than sp3 hybridized carbon atoms (25% s-character and 75% p-character). This makes sp2 hybridized carbon atoms more electronegative.
learn more about carbon atoms here.
https://brainly.com/question/30507533
#SPJ11
complete the bolded items in each box below
Answers
Show the bolded items and the boxes.
PLEASE HELP MEEE
this is due like in 10 min
how do you explain that 1 OF2 + H2o --> 1 O2 + 2 HF demonstrates that mass is conserved in a chemical l reaction
Answers
Answer:
In order to find the two statements, we must first define what the enthalpy of formation and the enthalpy of reaction mean.
Enthalpy of formation:
The change in enthalpy when one mole of substance is formed from its constituent elemetns at standard state.
Enthalpy of reaction:
The change in enthalpy when a reaction occurs and the reactants and products are in their standard states.
Now, we check the statements. The true ones are:
The Hrxn for C(s) + O₂(g) → CO₂(g) is the same as Hf for CO₂
This is true because the formation of carbon dioxide requires carbon and oxygen in their standard states.
The Hf for Br₂(l) is 0 kJ/mol by definition.
Because the bromine is present in its standard state, the enthalpy of formation is 0.
The Hrxn for the reaction 1.5H₂(g) + 0.5N₂(g) → NH₃(g) is the same as the Hf for NH₃(g)
The reactants and products are present in their standard state, and the reaction is the same as the one occurring during the formation of ammonia.
By using constants in an experiment, you can make sure that you are testing only one
A)experiment
B)variable
C)hypothesis
D)order
Answers
Experiment-the actual test
Variable-what you are testing
Hypothesis-a prediction
Which of the following is a non-polar molecule (have no permanent bond dipole moment)? Select the correct answer below: O CO2 be CO O CHO O CHO
Answers
CO₂ is a non-polar molecule. The correct answer is CO₂.
CO₂, which is carbon dioxide, is a non-polar molecule because it has a symmetrical shape and its bond dipoles cancel each other out. In CO₂, the carbon atom is bonded to two oxygen atoms. The molecule has a linear shape, with the carbon atom in the center and the oxygen atoms on either side.
The bond between the carbon atom and each oxygen atom is polar because oxygen is more electronegative than carbon, creating a partial negative charge on the oxygen atoms and a partial positive charge on the carbon atom. However, because the molecule is linear, the bond dipoles are equal in magnitude and opposite in direction, effectively canceling each other out.
This results in a non-polar molecule overall, with no permanent bond dipole moment. To summarize, CO₂ is a non-polar molecule because its bond dipoles cancel each other out due to its symmetrical linear shape. Hence, CO₂ is the correct answer.
You can learn more about non-polar molecules at: brainly.com/question/32290799
#SPJ11
the beta decay of cesium-137 has a half-life of 30.0 years. how many years must pass to reduce a 26 mg sample of cesium 137 to 5.7 mg?
Answers
Answer:
10 pts you say?
Explanation:
What property of a metal does the image represent
Answers
Answer:
malleable
Explanation:
The image represent in malleable property of metal.
The image possibly represents the photoelectric effect of a metal, which is when it emits electrons after being exposed to electromagnetic radiation. Metals are also characterized by physical properties such as conductivity, malleability, metallic luster, and metallic bonding.
Explanation:Based on your question, the image possibly represents the photoelectric effect, a key property of metals. This phenomenon occurs when a metal surface exposed to electromagnetic waves of a certain frequency absorbs radiation and emits electrons. These emitted electrons are called photoelectrons. Metals can also exhibit free electron model behavior, where electrons freely roam within the metal structure.
Metals possess unique physical properties like conductivity, malleability, and metallic luster. Malleability refers to the metal's ability to deform without breaking, while conductivity refers to the metal's ability to transfer heat or electricity. A metallic luster gives metals their characteristic shiny appearance.
Finally, metals are also known for their metallic bonding—a unique force that holds together the atoms within a metallic solid. Metallic bonding gives rise to many useful and varied bulk properties of metals.
Learn more about Properties of Metals here:https://brainly.com/question/33514448
#SPJ2
A beaker of water is placed in a large sealed jar that is attached to a vacuum pump. As air is pumped
out of the jar, the water begins to boil because --

Answers
Answer:
C
Explanation:
As air is pumped out of the jar, the water begins to boil because "the air pressure in the jar has been lowered until it is equal to the vapor pressure of the water."
What is pressure?The force is applied perpendicular to an object's surface per unit area across which that force is spread is known as pressure. The pressure gauge in relation to the higher pressures would be known as gauge pressure. Pressure is measured in a variety of ways.
What is vapor pressure?Equilibrium or vapor pressure The pressure exerted by a vapor in thermodynamic equilibrium including its condensed phases at a particular temperature in a closed system is known as vapor pressure.
The temperature where the vapor pressure of liquid water matches the ambient pressure is known as the boiling point of water. Because we know that pressure, temperature, as well as temperature, are directly related, water with a high boiling point will have a high boiling point, whereas water with a low boiling point will have a low boiling point.
When we maintain a vacuum (pressure lower than atmospheric pressure), water starts to boil at temperatures below 100 degrees Celsius.
Hence the correct answer will be option 3
To know more about air pressure here
https://brainly.com/question/25699778.
#SPJ2
Determine the final temperature of a mixture of 50 grams of water at 10 C added to 120 grams of water at 80 C. Show all work. Use the simulator to check your answer. (Hint: This is a weighted average.)
Answers
Answer: 59.41
Explanation:
The final temperature of the system has been 130\(\rm ^\circ C\).
Specific heat can be defined as the amount of heat required to raise the temperature of 1 g of substance by 1-degree celsius.
The amount of heat lost and the heat gained have been equal.
Heat = mass × specific heat × change in temperature
Thus,
mc\(\Delta\)T = mc\(\Delta\)T
50 g × (10 - x) = 120 × (x - 80)
500 - 50x = 120x - 9600
70x = 9100
x = 130
Thus, the final temperature of the system has been 130\(\rm ^\circ C\).
For more information about the final temperature of the system, refer to the link:
https://brainly.com/question/13639803
co2(g)+casio3(s)+h2o(l)→sio2(s)+ca(hco3)2(aq) express your answer as a chemical equation including phases.
Answers
Silicon dioxide (SiO2) solid and calcium bicarbonate (Ca(HCO3)2) aqueous are created when carbon dioxide (CO2) gas combines with calcium silicate (CaSiO3) solid and water (H2O) liquid.
The balanced chemical equation for the reaction is:
CO2(g) + CaSiO3(s) + H2O(l) → SiO2(s) + Ca(HCO3)2(aq)
This reaction occurs when calcium silicate (CaSiO3) and carbon dioxide (CO2) come into contact with water (H2O). Calcium bicarbonate (Ca(HCO3)2) in aqueous form and silicon dioxide (SiO2) in solid form are the byproducts of the reaction.
The phases denoted by the letters (g), (s), and (aq) in the equation are gas, solid, and aqueous, respectively.
A chemical reaction occurs when carbon dioxide gas (CO2), calcium silicate (CaSiO3), and water (H2O) are combined. Calcium silicate and carbon dioxide combine, resulting in the solid substance silicon dioxide (SiO2).
Calcium bicarbonate (Ca(HCO3)2) is produced in aqueous form from the remaining ingredients.
Phases are a significant source of data on the physical states of the reactants and products in the chemical equation. It specifies whether the reactants are in a liquid, gaseous, or solid phase at the time of the reaction.
Learn more about Carbon dioxide here:
brainly.com/question/3049557
#SPJ11
Please help it’s due today, I will give brainliest

Answers
the two middle ones is the answer
If you wanted to mix pure methane with water and end up with 90 gallons of 60% methane, how many gallons of each should you use?
You should use ________ gallons of water and _________ gallons of methane
Answers
To determine the amount of water and methane needed, we can set up a system of equations based on the desired composition of the mixture. you should use 36 gallons of water and 54 gallons of methane to obtain a mixture of 90 gallons with a methane concentration of 60%.
Let's assume x represents the number of gallons of water and y represents the number of gallons of methane. We have the following information: The total volume of the mixture is 90 gallons: x + y = 90. The mixture should be 60% methane: (y / (x + y)) * 100 = 60. Simplifying the second equation: y / (x + y) = 0.6. Now we can solve the system of equations: From equation 1, we can express x in terms of y: x = 90 - y. Substituting this into equation 2: y / ((90 - y) + y) = 0.6. Simplifying further: y / 90 = 0.6. Solving for y: y = 0.6 * 90. y = 54. Now we can find x using equation 1: x = 90 - y. x = 90 - 54. x = 36. Therefore, you should use 36 gallons of water and 54 gallons of methane to obtain a mixture of 90 gallons with a methane concentration of 60%.
To learn more about methane, https://brainly.com/question/31473733
#SPJ11
Question 15 of 25
What is the standard cell notation of a galvanic cell made with aluminum and
magnesium?
A. Al3+(aq) | Al(s) || Mg(s) | Mg2+(aq)
B. Mg2+(aq) | Mg(s) || Al(s) | A13+(aq)
C. Mg(s) | Mg2+(aq) || A13+(aq)| Al(s)
D. Al(s) | Al3+(aq) || Mg2+ (aq) | Mg(s)
SUBMIT
Answers
The standard cell notation of a galvanic cell made with aluminum and magnesium is option A. Al3+(aq) | Al(s) || Mg(s) | Mg2(aq).
The right side is the cathode and the left side is the anode. The cell is represented by the convention that the metal is written first, then the metal ions present in the electrolyte. And these two should be separated by a vertical line. Zinc becomes the cathode of the galvanic cell.
Galvanic cells consist of two different metal electrodes connected by a conductive solution electrolyte, which are also connected externally to complete an electrical circuit. Cell notation or cell representation in chemistry is a simple way of representing reactions in an electrochemical cell. The silver half-cell is reduced due to its high standard reduction potential. Tin half-cells are oxidized.
Learn more about Galvanic cells here:-https://brainly.com/question/28182115
#SPJ1
Answer: C
Explaination:
Suppose you are given three different solutions containing Na,PO4, Ba(NO3)2, and K,CO, respectively. Based on
the results of this lab and other reference materials, hypothesize about which combinations of these solutions
will produce insoluble precipitates. Based on your observations of the behavior of the compounds studied in this lab and in previous lessons what general statements can you make about the solubility of ionic compounds
containing Na+, Ba2+, K+, PO4-, NO3-, and CO3.
Answers
The solubility of ionic compounds depends on the nature of the ions and their charges.
Solubility of ionic compoundsIonic compounds containing Na+, K+, and NO3- ions are generally soluble in water because they have small ionic radii and weak ionic interactions. On the other hand, ionic compounds containing Ba2+, PO4-3, and CO3-2 ions tend to be less soluble in water because they have larger ionic radii and stronger ionic interactions.
Ba2+ and PO4-3 ions tend to form insoluble compounds, such as Ba3(PO4)2, while Ba2+ and CO3-2 ions can also form insoluble compounds, such as BaCO3. K+ and CO3-2 ions may also form an insoluble precipitate when combined with certain cations such as Ba2+. Overall, the solubility of ionic compounds can be influenced by factors such as temperature, pH, and the presence of other ions in the solution.
Learn more on ionic compounds here https://brainly.com/question/2687188
#SPJ1
N2(g) + H2(g) → NH3 If 2.70 grams of N2(g) reacts, how many grams of NH3 is produced
Answers
2.70 grams of N2 reacts to produce 3.28 grams of NH3.
Describe Chemical Equation?A chemical equation is a symbolic representation of a chemical reaction that shows the reactants and products involved and the ratios in which they combine. It is a concise way to describe a chemical reaction using chemical formulas and symbols. The reactants are written on the left side of the equation and the products on the right side, separated by an arrow pointing from left to right.
The general form of a chemical equation is:
Reactants → Products
In a chemical equation, the reactants are the substances that undergo a chemical change, and the products are the substances that are formed as a result of the chemical reaction. The coefficients before each reactant and product indicate the relative amounts of each substance involved in the reaction.
For example, the chemical equation for the reaction between hydrogen gas and oxygen gas to form water can be written as:
2H2 + O2 → 2H2O
This equation indicates that two molecules of hydrogen gas (H2) react with one molecule of oxygen gas (O2) to form two molecules of water (H2O).
The law of conservation of mass dictates that the total mass of the reactants must equal the total mass of the products in a chemical equation. Therefore, the number of atoms of each element must be the same on both sides of the equation. If necessary, coefficients can be adjusted to balance the equation.
Overall, chemical equations are a fundamental tool for understanding chemical reactions and predicting the outcomes of chemical reactions.
The balanced chemical equation for the reaction between nitrogen gas (N2) and hydrogen gas (H2) to form ammonia gas (NH3) is:
N2(g) + 3H2(g) → 2NH3(g)
This equation shows that 1 mole of N2 reacts with 3 moles of H2 to produce 2 moles of NH3.
To find the number of grams of NH3 produced when 2.70 grams of N2 reacts, we can use the following steps:
Calculate the number of moles of N2:
Molar mass of N2 = 28.02 g/mol
Number of moles of N2 = Mass of N2 / Molar mass of N2
= 2.70 g / 28.02 g/mol
= 0.0963 mol
Use the mole ratio from the balanced equation to find the number of moles of NH3 produced:
Number of moles of NH3 = Number of moles of N2 x (2 moles of NH3 / 1 mole of N2)
= 0.0963 mol x (2 mol NH3 / 1 mol N2)
= 0.1926 mol NH3
Calculate the mass of NH3 produced using the molar mass of NH3:
Molar mass of NH3 = 17.03 g/mol
Mass of NH3 produced = Number of moles of NH3 x Molar mass of NH3
= 0.1926 mol x 17.03 g/mol
= 3.28 g
Therefore, 2.70 grams of N2 reacts to produce 3.28 grams of NH3.
To know more about moles visit:
https://brainly.com/question/15209553
#SPJ1
g stagflation is a combination of A. Falling GDP and falling prices. B. Recession and inflation. C. Rising GDP and rising prices. D. Increasing prices in the stocks and agricultural markets.
Answers
B. Recession and inflation. Stagflation is an economic phenomenon characterized by a combination of stagnant economic growth (recession) and high inflation.
It is a situation where an economy experiences a period of low or negative GDP growth, high unemployment, and rising prices. This combination is considered unusual because in typical economic conditions, recessions are associated with low inflation or even deflation. Stagflation poses challenges for policymakers as traditional measures to stimulate economic growth, such as monetary easing, may worsen inflationary pressures.
learn more about :- stagflation here
https://brainly.com/question/29552947
#SPJ11
what bond(s) is (are) disrupted in the presence of water?
Answers
In the presence of water, ionic and hydrogen bonds can be disrupted.
Water is a polar molecule, meaning it has a partial positive charge on one end and a partial negative charge on the other end.
This polarity allows water to interact with other polar molecules, including ionic compounds and molecules containing hydrogen bonds.
Ionic compounds are held together by strong electrostatic forces between positively and negatively charged ions.
In the presence of water, the partial charges on the water molecule can attract and surround the ions, weakening the electrostatic forces and causing the ionic compound to dissociate into its component ions.This is why ionic compounds dissolve readily in water.
Hydrogen bonds are a type of intermolecular force that forms between a hydrogen atom bonded to an electronegative atom and another electronegative atom in a different molecule.
Water molecules can form hydrogen bonds with other polar molecules, and in the presence of water, these hydrogen bonds can be disrupted as water molecules compete for hydrogen bonding partners. This can affect the solubility and reactivity of molecules containing hydrogen bonds.
Visit here to learn more about Molecules:
brainly.com/question/475709
#SPJ11
Which of the following about a balanced chemical reactions is
NOT true?
O A properly written chemical equation will include the states of matter
for every substance in the reaction.
O The number of atoms of each element must be the same for both
reactants and products.
A balanced chemical equation must follow the Law of Conservation
of Matter
A balanced chemical equation must always include coefficients on
every reactant and product.
Answers
Answer:
A balanced chemical equation must always include coefficients on every reactant and product.
Explanation:
A balanced chemical equation does not need to include coefficients on every reactant and product.
For example, below is a balanced chemical equation in which the reactants and the products have no coefficients whatsoever:
NaOH(aq) + HCl (aq) -----> NaCl (s) + H2O (l)
Of course, a properly written chemical equation must include the states of matter of all the substances in the reaction and the number of atoms of each element must balance both in the reactant and product sides of the equation. Generally, a balanced chemical equation must obey the law of conservation of matter which opines that matter can neither be created nor destroyed but can only be converted from one form to another.
Hence, that a balanced chemical equation must always include coefficients on every reactant and product is not true.
The two following body systems work closely together: the __________ brings oxygen into the body and disposes of carbon dioxide and the ___________ circulates oxygen throughout the body and keeps blood fresh.
Answers
2.) circulatory system
When the phenol shown below is treated with KOH, it forms a product whose IR spectrum does not show an absorption in the 3200-3600 cm-1 region. Propose a structure for the product.?
Answers
Answer:
Explanation:
The first attached diagram shows the proposed phenol that is being treated with KOH to form Oxonine (CₐHₐO).
Reaction of Arylhalide after treatment with KOH lead to the displacement of Bromine atom with the hydrogen from the hydroxyl (OH) substituent , then forming HBr , leaving the oxy (-O) atom alone to form an Oxonine compound.
Therefore the propose structure for the product (Oxonine) CₐHₐO is shown in the second diagram attached below.


calculate the amount of parent isotope that remaining after 1, 2, 3, and 4 half-lives. include 4 decimal places in your answers. parent present: 1.0000 unit (i.e., all of the parent isotope is still present) after 1 half-life: 0.5000 units
Answers
For each half-life, the total amount of the isotope is divided by 2.
Therefore, the amount of parent isotope that remains after:
1 half-life is ½= 0.5
2 half-lives= 2/4= 0.5
3 half-lives= 3/6= 0.5
What is an isotope?
Isotopes are two or more atoms sharing the same atomic number and chemical element but have distinct nucleon numbers (mass numbers) as a result of having a different number of neutrons in their nuclei. The word "isotope" is derived from the Greek words "isos" and "topos," both of which imply "the same location." As a result, the name refers to the fact that various isotopes of a given element occupy the same spot on the periodic table. Scottish physician and author Margaret Todd first used it in a recommendation in 1913.To know more about isotopes, click the link given below:
https://brainly.com/question/11394246
#SPJ4
electronegativity for boron is 2.0, and electronegativity for fluorine is 4.0. which type of bond forms between boron (b) and fluorine (f) in bf3?
Answers
Electronegativity for boron is 2.0, and electronegativity for fluorine is 4.0. which type of bond forms between boron (B) and fluorine (F) in BF₃ is non polar covalent bond.
The BF₃ is the covalent molecule. the electronegativity for boron is 2.0 and the electronegativity for the fluorine is 4.0 . the boron has 3 valence electrons and need 5 more to complete the octet and fluorine has 7 valence electrons . thus boron form three covalent bond with the atom of fluorine and try to complete the octet.
The BF₃ is a triangular planar molecule. the dipole moment in the BF₃ molecule is zero therefore BF₃, boron trifluoride is a non polar covalent molecule.
To learn more about electronegativity here
https://brainly.com/question/19374222
#SPJ4
a solution is 1.5 m nabr. what is the weight percent of nabr in this solution? what is the mole fraction of nabr in this solution?
Answers
The weight percent of NaBr in this solution is 150% and the mole fraction of NaBr in this solution will be 1.5.
Weight percent is a measure of the amount of solute (in this case, NaBr) in a solution relative to the total weight of the solution.
Weight percent (w/w) = (mass of solute / total mass of solution) x 100
In this case, the solution is 1.5 M NaBr, so we can calculate the mass of the solute:
mass of solute = molarity x molecular weight of solute
mass of solute = 1.5 M x 102 g/mol
mass of solute = 153 g
The total mass of the solution calculated as:
total mass = mass of solute / molarity
total mass = 153 g / 1.5 M
total mass = 102 g
Now we calculate the weight percent:
Weight percent (w/w) = (mass of solute / total mass of solution) x 100
Weight percent (w/w) = (153 g / 102 g) x 100
Weight percent (w/w) = 150%
The mole fraction of a solute is the ratio of the number of moles of the solute to the total number of moles in the solution.
The number of moles of NaBr can be calculated as:
number of moles of NaBr = mass of NaBr / molecular weight of NaBr
number of moles of NaBr = 153 g / 102 g/mol
number of moles of NaBr = 1.5 mol
And the total number of moles in the solution can be calculated as:
total number of moles = number of moles of NaBr / molarity
total number of moles = 1.5 mol / 1.5 M
total number of moles = 1.0 mol
Now we calculate the mole fraction:
Mole fraction = 1.5 mol / 1.0 mol
Mole fraction = 1.5
To know more about mole fraction here
https://brainly.com/question/29808190
#SPJ4
Describe the overall enthalpy of the chemical reactants compared to the enthalpy of the chemical products in the combustion of propane.
Answers
Answer:
A thermochemical equation for the combustion of propane (C3H8)(C3H8) is written as follows:
C3H8(l)+5O2(g)→3CO2(g)+4H2O(g);ΔH∘rxnC3H8(l)+5O2(g)→3CO2(g)+4H2O(g);ΔHrxn∘ = -2202.0 kJ/mol
The value given for ΔH∘rxnΔHrxn∘ means that:
a. the reaction of one mole of propane absorbs 2202 kJ of energy from the surroundings.
b. the reaction is endothermic.
c. the enthalpy of formation of propane is 2202 kJ/mol.
d. the reaction of one mole of propane releases 2202 kJ of energy to the surroundings.
e. None of these.
Iron has a density of 5.5 g/mL. What would be the volume of a 1500 g sample?
Answers
Answer:
volume = 272.7 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{Density} \)
From the question
mass of Iron = 1500 g
Density = 5.5 g/mL
Substitute the values into the above formula and solve
That's
\(volume = \frac{1500}{5.5} \\ = 272.727272...\)
We have the final answer as
volume = 272.7 mLHope this helps you