At about ph 7 in most cells, what happens to the amino group on an amino acid?.
Answers
At a pH of around 7 in most cells, the amino group on an amino acid will be protonated, meaning it will have a positive charge. This is because the pH is below the pKa of the amino group, which is around 9-10, making it a stronger acid than the surrounding environment. As the pH increases above the pKa, the amino group becomes deprotonated and loses its positive charge.
Related Questions
Fill in the lone pairs needed to give the main group elements (except hydrogen) an octet. Acrylonitrile is a starting material used to manufacture synthetic Orlon and Acrilan fibers. Cysteine is an amino acid used to synthesize proteins.
Answers
Acrylonitrile is a molecule used to manufacture synthetic Orlon and Acrilan fibers, and its chemical formula is C3H3N. Cysteine is an amino acid used to synthesize proteins, and its chemical formula is C3H7NO2S.
To fill in the lone pairs needed to give the main group elements (except hydrogen) an octet, we need to examine the electron configuration of the atoms and determine the number of valence electrons available. Then, we can use Lewis structures to show the bonding and nonbonding electrons and determine the number of lone pairs required to complete the octet.
The central carbon atom has four valence electrons, while the nitrogen atom has five valence electrons. The carbon atoms are each bonded to two other atoms (one hydrogen and one carbon), and the nitrogen atom is bonded to one carbon atom and has one lone pair.
To complete the octet of the carbon and nitrogen atoms, we need to add one and two lone pairs, respectively. The Lewis structure of acrylonitrile with the added lone pairs would look like this:
H H
| |
C==C- - - C≡N
| |
H lone pair
The central carbon atom has four valence electrons, while the sulfur atom has six valence electrons. The carbon and nitrogen atoms are each bonded to two other atoms (hydrogen and other carbon/nitrogen atoms), and the sulfur atom is bonded to two carbon atoms and has one lone pair.
To complete the octet of the carbon, nitrogen, and sulfur atoms, we need to add one, two, and two lone pairs, respectively. The Lewis structure of cysteine with the added lone pairs would look like this:
H H
| |
H-C-C-C- - -N
| |
H H
|
S
/
C C
| |
H H
To learn more about Acrylonitrile
https://brainly.com/question/13731887
#SPJ4
Which type of reaction occurs in the following?
2HgO(s) Right arrow. 2Hg(l) + O2(g)
a decomposition reaction in which a metal is reduced
an acid-base neutralization reaction to produce oxygen gas
a displacement reaction involving two metals
a combustion reaction involving burning a metal in oxygen
Answers
Answer:
decomposition
Explanation:
the reactants turn into two different products, thus the reaction is decomposition.
AB -> A + B
Answer:
A: a decomposition reaction in which a metal is reduced
Explanation:
HgO is actually the metal Mercury and in the equation Mercury is being decomposed into the elements Hg and O. (the element for Mercury and Oxygen) So the equation they give you is a decomposition.

a buffer solution contains 0.301 m khco3 and 0.288 m k2co3. if 0.0273 moles of sodium hydroxide are added to 125 ml of this buffer, what is the ph of the resulting solution ? (assume that the volume does not change upon adding sodium hydroxide)
Answers
The pH of the resulting solution is 9.96 that can be calculated by using the using the pOH values.
Buffer Solution is a water solvent primarily based totally answer which includes a combination containing a vulnerable acid and the conjugate base of the vulnerable acid, or a vulnerable base and the conjugate acid of the vulnerable base. They face up to a alternate in pH upon dilution or upon the addition of small quantities of acid/alkali to them.
Millimoles of NH3 = 0.316 x 250 = 79
Millimoles of NH4Br = 0.339 x 250 = 84.75
Millimoles of KOH = 57.3
pOH = pKb + log [salt - C / base + C]
= 4.74 + log [84.75 - 57.3 / 79 + 57.3]
= 4.04
pH + pOH=14
pH=14-4.04
pH = 9.96
To learn more about pH check the link below:
https://brainly.com/question/172153
#SPJ4
What are 4 things an element can do with electrons
Answers
Answer:Each shell can hold a maximum number of electrons. moving through the elements in the periodic table, each atom has one more electron than the last because the number of electrons is the same as the atomic number. Electrons occupy the shells in order, starting with the shell that is nearest the nucleus.
Explanation:
The empirical formula of a compound is C5H7O. What is the molecular formula if the molecular mass is 249.3 g/mol.
Answers
Answer:The molecular formula is C5 H10 O5 .
Explanation:
Fill in the number of valence electrons of the elements listed: Li
______ I________ B________ P_______ Mg ________
Answers
Answer:
Explanation: It's simple work really but the answers are
Li : 1
I : 7
B : 3
P : 5
Mg : 2
The number of valence electrons in Li, I, B, P, and Mg are 1, 7, 3, 5, and 2 respectively.
What is a valence electron?Valence electrons can be defined as the electrons filled in the outermost shell of an atom while the electrons in the inner electron shell are called core electrons. Lewis structures can be helpful to find the number of valence electrons and knowing the types of chemical bonds.
Valence electrons are occupied in different electron shells and these electrons are responsible for the interaction between atoms and participate in the formation of chemical bonds.
The Lithium element with 2s¹ valence shell configuration so it has one valence electron.
The Iodine element with 5s²5p⁵ valence shell configuration so it has seven valence electrons.
The Boron element with 2s²2p¹ valence shell configuration so it has three valence electrons.
The phosphorous with 3s²3p³ valence shell configuration so it has five valence electrons.
The Magnesium with 3s² valence shell configuration so it has two valence electrons.
Learn more about valence electrons, here:
brainly.com/question/18612412
#SPJ2
If energy is shown on the product side of the equation, then the
1.
reaction
A.
is exothermic.
B.
is endothermic.
C.
does not require any activation energy.
D.
does not include
any
other product.
Answers
Answer:
is exothermic
Explanation:
releases energy to the atmosphere
Bradley is planning to publish a cookbook. It will include 150 recipes, with one recipe on each page. They also decide to divide the book into three sections: vegetarian dishes, meat dishes, and desserts
Answers
A cookbook is a small book that contains the recipes for preparing different types of meals.
What is a cookbook?A cookbook is a small book that contains the recipes for preparing different types of meals sometimes including continental dishes. The recipes in a cookbook are often invaluable for chefs.
A cookbook may contain the recipe for different types of diet such as;
vegetarian dishes, meat dishes, dessertsLearn more about cookbook:https://brainly.com/question/26237068?
#7) How many waves are in this picture?

Answers
Answer:
4
Explanation:
Answer:
B.
Explanation:
I don't know much about this subject, but it seems that a wave is when the line is above the line in the middle.
The invention of the microscope led to the discovery of the cell by Robert Hooke. While looking at cork, Hooke observed the box-shaped structures, which he called "cells" because they reminded him of the cells (rooms) found in monasteries. This discovery led to the development of the cell theory. Credit for developing the cell theory is usually given to two scientists: Theodor Schwann and Matthias Schleiden. The cell theory was then proposed by Theodor Schwann in 1839. There are three parts to this theory.
Examine each of the statements below. Which of these is part of the cell theory? Select ALL that apply.
A.Energy flow occurs within cells.
B.All cells arise only from pre-existing cells.
C.DNA is passed between cells during cell division.
D.All living things are made of cells.
The cell is the basic unit of life.
Answers
The statements that are part of the cell theory are:
D. All living things are made of cells.
E. The cell is the basic unit of life.
A. All cells arise only from pre-existing cells.
Cell theory refers to energy flow within cells, which is a concept related to cellular metabolism but not a fundamental part of the cell theory. Option C is also not part of the cell theory, as it refers to the passing of DNA between cells during cell division, which is a biological process but not a defining feature of cells or the cell theory.
Learn more about cell theory, here:
https://brainly.com/question/1468725
#SPJ1
based on figure 1, which of the following statements best predicts the effect that a change from a moderately acidic environment (phph near 6) to a basic environment will have on peroxidase activity?
Answers
Peroxidase activity increases and then decreases due to a change from a moderately acidic environment (pH near 6) to a basic environment.
What is peroxidase activity?Peroxidase is an enzyme found in a wide variety of organisms, from plants to humans to bacteria. Its function is to decompose hydrogen peroxide (H₂O₂). Hydrogen peroxide is one of the toxins produced as a by-product of using oxygen to breathe. (The fact that hydrogen peroxide is toxic makes it useful in first aid kits.
Peroxidase activity is strongly influenced by pH factors. Peroxidase works best at pH 7, but increasing or decreasing pH adversely affects its activity. Therefore, when moving from a weakly acidic environment close to pH 6 to a basic one, the peroxidase activity increases as the neutral environment approaches and decreases as the basic environment approaches, so that the peroxidase activity varies with the pH of the solution.
To know more about peroxidase, visit:
https://brainly.com/question/14870911
#SPJ1
The complete question is as follows:
Researchers investigated the influence of environmental pH on the activity of peroxidase, an enzyme that catalyzes the conversion of hydrogen peroxide to water and oxygen gas. In an experiment, the researchers added a hydrogen peroxide solution containing guaiacol to several identical test tubes and adjusted the solution in each test tube to a different pH . The researchers included the guaiacol because it caused the solutions to change color as the reactions proceeded, which the researchers relied on for measuring reaction rates. Finally, the researchers added the same amount of peroxidase to each test tube and measured the rate of each reaction at 23°C . The results of the experiment are represented in Figure 1.
Based on Figure 1, which of the following statements best predicts the effect that a change from a moderately acidic environment ( pH near 6) to a basic environment will have on peroxidase activity?
answer choices
Peroxidase activity will decrease.
Peroxidase activity will increase.
Peroxidase activity will stay the same.
Peroxidase activity will increase at first and then decrease.
Compound A, an alkyl bromide with chemical formula , reacts with to yield compound B. Compound B in turn reacts with (1) , (2) , to yield compound C. Compound C reacts with to yield compound D. Propose a structure for compound D that is consistent with the following predicted 1H-NMR spectrum.
Answers
Compound B will be propene as a result of an elimination reaction involving potassium tert-butoxide and an alkyl halide. In proton NMR, alkenes exhibit signals in the 5–6 ppm range.
What are Elimination Reactions?Elimination reactions are one sort of reaction that are mostly utilized to convert saturated compounds (organic molecules that contain single carbon-carbon bonds) to unsaturated compounds (compounds that feature double or triple carbon-carbon bonds). Additionally, it plays a significant role in the production of alkenes.
The elimination response is composed of the following three major events:
Proton exclusion. The synthesis of a C-C pi bond. As a result, the relationship between the departing group's members has grown increasingly distant.To learn more about Elimination reactions visit:
https://brainly.com/question/14693649
#SPJ4
Why is degrees used in measuring angles and temperature?.
Answers
PLEASE HELP DUE IN 15!!!!!!!!!!!! WILL GIVE BRAINLIEST!!
Question 17: 2C2H6 + 7O2 ---> 4CO2 + 6H20
How many moles of O2 are needed to completely react with 4.50 moles of C2H6 according to the above equation?
a. 5.8 moles
b. 3.11 moles
c. 1.29 moles
d. 63.0 moles
Answers
Explanation:
n=,4.5 moles C2H6
the ratio 2:7
4.5:x
x=15.75 moles
What is the connection between physical properties and physical changes
Answers
Answer:
Info from G00gle:Physical changes are related to physical properties since some measurements require that changes be made. Melting Point: As solid matter is heated it eventually melts or changes into a liquid state at the melting point. Ice (a solid form of water) melts at 0 oC and changes to the liquid state.
Explanation:
Mark me brainliest
Answer:
the answer is A
Explanation:
Cracking of long saturated hydrocarbon chain molecule C40H82 produces 3 octane molecules and the rest as ethane molecules. How many moles of hydrogen are needed to crack one mole of this long hydrocarbon chain? Give your answer in whole numbers.
Answers
To determine the number of moles of hydrogen needed to crack one mole of the long saturated hydrocarbon chain (C40H82), we can analyze the reactants and products involved in the cracking reaction.
The cracking reaction is given as: C40H82 -> 3 C8H18 + n C2H6. From the equation, we can see that one mole of the long hydrocarbon chain (C40H82) produces three moles of octane (C8H18) and n moles of ethane (C2H6). Since the cracking process involves breaking the carbon-carbon bonds and forming new carbon-hydrogen bonds, the number of hydrogen atoms in the products should remain the same as in the reactant.
The long hydrocarbon chain (C40H82) contains 82 hydrogen atoms, and the products, 3 moles of octane (C8H18), contain (3 moles) * (18 hydrogen atoms/mole) = 54 hydrogen atoms. Therefore, the number of moles of hydrogen needed for cracking one mole of the long hydrocarbon chain can be calculated as: Number of moles of hydrogen = 82 - 54 = 28 moles. Hence, 28 moles of hydrogen are required to crack one mole of the long saturated hydrocarbon chain (C40H82).
To learn more about number of moles click here: brainly.com/question/20370047
#SPJ11
In what order should atoms be balanced in a hydrocarbon combustion
reaction?
A. Oxygen, hydrogen, carbon
B. Carbon, hydrogen, oxygen
C. Hydrogen, oxygen, carbon
D. Oxygen, carbon, hydrogen
Answers
Answer:
B . Carbon hydrogen oxygen
Explanation:
I'll hope work it
Is it coating iron pipe with Zinc or connecting a zinc rod to a
iron pipe, which is advantageous to protect the Fe surface from
undergoing corrosion? Justify the answer
Answers
Connecting a zinc rod to an iron pipe offers advantages in protecting the iron surface from corrosion. The zinc acts as a sacrificial anode, corroding in place of the iron and providing uniform and extended protection to the entire iron pipe.
Connecting a zinc rod to an iron pipe is advantageous to protect the iron (Fe) surface from undergoing corrosion. This process is known as cathodic protection, where the zinc acts as a sacrificial anode. Here's the justification for this answer:
Galvanic Protection: When a zinc rod is connected to an iron pipe, it creates a galvanic cell. Zinc is more reactive than iron, so it acts as the anode, sacrificing itself to protect the iron pipe (cathode). The zinc corrodes instead of the iron, thereby providing protection to the iron surface.Sacrificial Anode: Zinc has a higher electrochemical potential than iron, making it more susceptible to corrosion. This means that zinc will preferentially corrode instead of the iron pipe. By connecting a zinc rod, the zinc sacrificially corrodes, protecting the iron from corrosion. Uniform Protection: Connecting a zinc rod provides uniform protection to the entire iron pipe surface. As long as the zinc rod is in contact with the iron pipe, it will continuously provide cathodic protection along the entire length of the pipe. Extended Protection: The sacrificial zinc anode can provide protection for an extended period before it gets fully consumed. Once the zinc is depleted, it can be replaced with a new zinc rod to continue the protection.Read more on corrosion here: https://brainly.com/question/489228
#SPJ11
Complete combustion of 8.00 g of a hydrocarbon produced 25.7 g of CO2 and 8.77 g of H2O. What is the empirical formula for the hydrocarbon? Insert subscripts as necessary.
Answers
Complete combustion of 8.00 g of a hydrocarbon produced 25.7 g of CO₂. The empirical formula for the hydrocarbon is C₄H₇.
What is the empirical formula?The empirical formula of the molecule is the simplest whole number of atoms of a compound.
Moles of C in the compound: 27.8 g CO2 x 1 mol CO2/44 g x 1 mol C/mole CO2 = 0.632 moles of C
Moles of H in the compound: 9.96 g H2O x 1 mol H2O/18 g x 2 mol H/mol H2O = 1.11 moles of H
mass C = 0.632 mol C x 12 g/mol = 7.58 g C
mass H = 1.11 mol H x 1 g/mol = 1.11 g H
Sum = 8.69 g total mass (close enough to 8.70 so our assumption of no oxygen is correct)
To find the lowest whole number of moles, we can divide both by the lowest value (0.632) to obtain.
moles C = 0.632/0.632 = 1.0
moles H = 1.11/0.632 = 1.75
Now to get a whole number for H we can multiply both by 4 to obtain
moles C = 4
moles H = 7
Thus, the Empirical formula is C₄H₇.
To learn more about the Empirical formula, refer to the below link:
https://brainly.com/question/14044066
#SPJ1
help me ASAP PLEASEEEEEEEEEEEEE!!!!!!!!!!!!!!!!!!

Answers
Answer:
Cells
Explanation:
100 cm³ of a gas at 27°C is cooled to 20°C at constant pressure .Calculate the volume of gas at 20°C.
Answers
According to Charle's law, the volume of the given mass of a gas is directly proportional to its absolute temperature provided that the pressure is constant. Mathemically;
\(\begin{gathered} V\alpha T \\ V=kT \\ k=\frac{V}{T} \\ k=\frac{V_1}{T_1}=\frac{V_2}{T_2} \end{gathered}\)where;
V1 and V2 are the initial and final volume of the gas
T1 and T2 are the initial and final temperatures of the gas (in Kelvin)
Given the following parameters:
\(\begin{gathered} V_1=100\operatorname{cm}^3 \\ T_1=27^0C=27+273=300K \\ T_2=20^0C=20+273=293K \\ V_2=\text{?} \end{gathered}\)Substitute the given parameters into the formula;
\(\begin{gathered} V_2=\frac{V_1T_2}{T_1}^{} \\ V_2=\frac{100\times293}{300} \\ V_2=\frac{29300}{300} \\ V_2=\frac{293}{3} \\ V_2=97.67\operatorname{cm}^3 \end{gathered}\)Therefore the volume of the gas at 20°C is approximately 97.67cm³
Energy in vs Energy out = Energy balance. Explain this concept, give examples and provide support for your explanation.
Answers
The concept of energy balance refers to the equilibrium between the energy input into a system and the energy output from that system. It is based on the principle of conservation of energy, which states that energy cannot be created or destroyed but can only be transferred or transformed from one form to another.
In terms of human energy balance, it involves the energy intake from food and beverages (energy in) and the energy expenditure through basal metabolic rate, physical activity, and other bodily processes (energy out). When the energy intake matches the energy expenditure, there is an energy balance. However, when there is an imbalance, either an excess or deficit of energy, it can lead to weight gain or weight loss, respectively.
For example, if a person consumes 2000 calories (energy in) through their diet and expends 2000 calories (energy out) through their daily activities and bodily functions, they maintain an energy balance. This means that the energy intake is equal to the energy expenditure, and their weight remains stable.
On the other hand, if a person consumes 2500 calories (energy in) but only expends 2000 calories (energy out), there is a positive energy balance. The excess energy is stored in the body as fat, leading to weight gain over time.
Conversely, if a person consumes 1500 calories (energy in) but expends 2000 calories (energy out), there is a negative energy balance. The body needs to compensate for the energy deficit by utilizing stored energy reserves, such as fat, resulting in weight loss.
Support for the concept of energy balance comes from scientific studies on weight management and obesity. It has been shown that maintaining an energy balance is crucial for weight maintenance, while sustained positive or negative energy balances can lead to weight changes. Additionally, energy balance plays a role in various physiological processes, including metabolism, hormone regulation, and overall health.
By understanding and managing energy balance, individuals can make informed decisions regarding their diet, physical activity, and lifestyle to achieve and maintain a healthy weight and overall well-being.
To know more about energy balance, click here, https://brainly.com/question/31922451
#SPJ11
HELP SOON POSSIBLEEEE

Answers
Answer:
1. atom
2. different
3. compounds
4. joined
5. separated
--
1. NT
2. ST
3. AT
--
1. subatomic
2. negative
3. Protons
4. neutrons
5. Nucleus
6. Rutherford
7. positive
8. electrons
--
1. T
2. T
3. F
4. T
Explanation:
look at the answer
please help me out i will give you brainlist. 0.500 is wrong

Answers
============================================
Work Shown:
Using the periodic table, we see that
1 mole of carbon = 12 grams1 mole of oxygen = 16 gramsThese are approximations and these values are often found underneath the atomic symbol. For example, the atomic weight listed under carbon is roughly 12.011 grams. I'm rounding to 2 sig figs in those numbers listed above.
So 1 mole of CO2 is approximately 12+2*16 = 44 grams. The 2 is there since we have 2 oxygens attached to the carbon atom.
-------------------
Since 1 mole of CO2 is 44 grams, we can use that to convert from grams to moles.
11.0 grams of CO2 = (11.0 grams)*(1 mol/44 g) = (11.0/44) mol = 0.250 mol of CO2
In short,
11.0 grams of CO2 = 0.250 mol of CO2
This is approximate.
We don't need to use any of the information in the table.
Answer:
.250
Explanation:
why hot water is hot on touching?
Answers
Thermal Energy
When a rise in temperature causes atoms and molecules to move faster and collide with each other. The energy that comes from the temperature of the heated substance is called thermal energy.
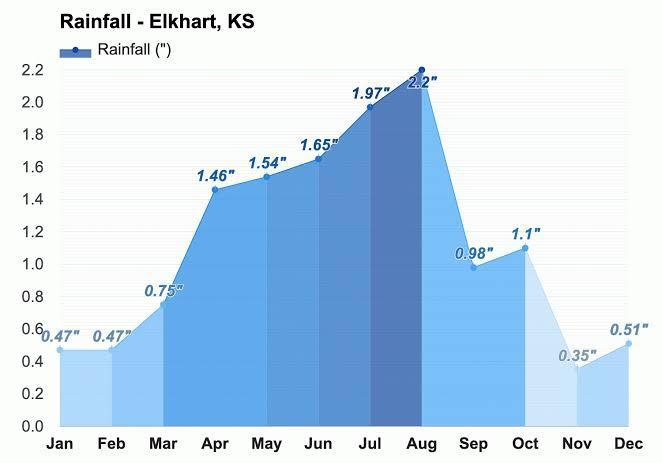
create a bar graph that shows the total yearly precipitation for elkhart kansas
Answers
The total yearly precipitation for elkhart kansas is attached in the form of graph below.
What is precipitation?Precipitation is defined as any liquid or frozen water which forms in the atmosphere and then gets received on Earth.It is one of the most important steps of the water cycle.
Precipitation takes place in form of clouds when water vapor gets accumulated in clouds and they get bigger and heavy, when the clouds become heavy enough they fall to the land in the form of rain.f a cloud is present at higher altitudes , the water present in the clouds freezes and fall to the ground in form of snow,hail.
Learn more about precipitation ,here:
https://brainly.com/question/18109776
#SPJ1
Can someone walk me through this question??
The Percent yield for the reaction PCl3 +Cl2 --> PCl5 is 83.2%. What mass of PCl5 is expected from the reaction of 73.7% of PCl3 with excess chlorine?
Answers
The mass of \(PCl_5\) expected from the reaction of 73.7% of \(PCl_3\) with excess chlorine based on 83.2% percent yield would be 92.99 grams.
Percent yieldFrom the balanced equation of the reaction:
\(PCl_3 +Cl_2 -- > PCl_5\)
The ratio of the moles of \(PCl_3\) that reacts to that of \(PCl_5\) that is produced is 1:1.
73.7% of \(PCl_3\) = 73.7/137.33
= 0.5367 moles
Since the mole ratio is 1:1, the equivalent mole of \(PCl_5\) will also be 0.5367 moles.
Mass of 0.5367 moles \(PCl_5\) = 0.5367 x 208.24
= 111.7624 grams
But the reaction has only 83.2% yield:
111.7624 x 83.2% = 92.99 grams
Thus, the mass of \(PCl_5\) expected from the reaction will be 92.99 grams.
More on stoichiometric problems can be found here; https://brainly.com/question/27287858
#SPJ1
Which element is located in period 4?
Silicon (SI)
Iron (Fe)
Beryllium (Be) Zirconium (Zr)
Answers
Answer:
i beleive iron
Explanation:
Determine if the individual bonds in each compound are polar or nonpolar.
Include electronegativity values and the difference to show your work.
EXAMPLE: Se=2.4 O= 3.5 3.5-2.4=1.1 Polar
1. SCl2
2. H2S
3. CF4
4. PC15
5. CHA
6. CaBr2
7. SF2
8. CO2
9. NH3
10. Na s
Answers
Answer:
See explanation
Explanation:
For SCl2
S = 2.8, Cl = 3.16 difference = 0.4 polar
H2S
H = 2.2 S= 2.8 difference = 0.6 polar
CF4
C = 2.55, F= 3.98 difference = 1.43
This compound is nonpolar irrespective of the difference in electronegativity because the molecule is symmetrical
PCl5
P= 2.19, Cl= 3.16 difference = 0.97
This compound is nonpolar irrespective of the difference in electronegativity because the molecule is symmetrical
CH4
C = 2.55 H = 2.2 difference = 0.35 the molecule is nonpolar
CaBr2
Ca= 1 Br= 2.96 difference = 1.96
The compound is not just polar but ionic in nature due to large electronegativity difference between the bonding atoms
SF2
S = 2.8 F= 3.98 difference = 1.18
The molecule is polar
CO2
C= 2.55 O= 3.44 difference = 0.89
This compound is nonpolar irrespective of the difference in electronegativity because the molecule is symmetrical
NH3
N= 3.04 H = 2.2 difference =0.84
The molecule is polar
NaS
Na= 0.93 S = 2.8 difference = 1.87
The compound is not just polar but ionic in nature due to large electronegativity difference between the bonding
a radioactive substance has a half life of 8 minutes. how long will it take for 93.75% of this chemical to decay
Answers
To determine how long it will take for 93.75% of a radioactive substance to decay, we can use the concept of half-life. The half-life of a radioactive substance is the time it takes for half of the initial quantity to decay.
Given that the half-life of the substance is 8 minutes, we can calculate the number of half-lives it would take for the substance to decay to 93.75% of its original amount.
If we start with 100% of the substance, after one half-life (8 minutes), we would have 50% remaining. After two half-lives (16 minutes), we would have 25% remaining. After three half-lives (24 minutes), we would have 12.5% remaining. After four half-lives (32 minutes), we would have 6.25% remaining. And after five half-lives (40 minutes), we would have 3.125% remaining.
Therefore, it would take approximately 40 minutes for 93.75% of the substance to decay. This is because after five half-lives, we are left with 3.125% of the original amount, which is the closest value to 93.75%.
It's important to note that although the majority of the decay occurs within the first few half-lives, radioactive decay continues indefinitely, and a small amount of the substance will always remain.
In conclusion, if a radioactive substance has a half-life of 8 minutes, it will take approximately 40 minutes for 93.75% of the substance to decay.
For such more question on substance
https://brainly.com/question/29108029
#SPJ11