At 40∘∘ Celsius, the value of Kw is 2.92×10−14.
a.) Calculate the [H+][H+] and [OH−][OH−] in pure water at 40∘∘ Celsius.
b.) What is the pH in pure water at 40∘∘ Celsius?
c.) If [OH−][OH−] is 0.18 M , what is the pH?
Answers
a.) At 40°C, the values of [\(H^+\)] and [\(OH^-\)] in pure water are [\(H^+\)] = 1.71×\(10^{-7}\) M and [\(OH^-\)] = 1.70×\(10^{-7}\) M, respectively.
b.) The pH of pure water at 40°C is 6.77.
c.) If the [\(OH^-\)] concentration is 0.18 M, the pH of the solution is 12.79.
The value of Kw (the ion product constant for water) at 40°C is 2.92×\(10^{-14}\).
a) Since pure water is neutral, [\(H^+\)][\(OH^-\)] = Kw. Therefore, we can find [\(H^+\)] and [\(OH^-\)] as follows:
[\(H^+\)][\(OH^-\)] = Kw
\([H^+]^2\) = Kw = Kw
[\(H^+\)] = (\(\sqrt{Kw}\)) = (\(\sqrt{2.92\times10^{-7}}\) = 1.71×\(10^{-7}\) M
[\(OH^-\)] = Kw/[\(H^+\)] = 2.92× / 1.71×\(10^{-7}\) M = 1.70×\(10^{-7}\) M
Therefore, the values of [\(H^+\)] and [\(OH^-\)] in pure water at 40°C are [\(H^+\)] = 1.71×\(10^{-7}\) M and [\(OH^-\)] = 1.70×\(10^{-7}\) M.
b) The pH is defined as the negative logarithm of the hydrogen ion concentration, pH = -log[\(H^+\)]. Therefore, we can find the pH of pure water at 40°C as follows:
pH = -log[\(H^+\)] = -log(1.71×\(10^{-7}\)) = 6.77
Therefore, the pH of pure water at 40°C is 6.77.
c) If [\(OH^-\)] = 0.18 M, we can find [\(H^+\)] as follows:
[\(H^+\)][\(OH^-\)]= Kw
[\(H^+\)] = Kw/[\(OH^-\)] = 2.92×\(10^{-14}\) / 0.18 M = 1.62×\(10^{-13}\) M
The pH is then:
pH = -log[\(H^+\)] = -log(1.62×\(10^{-13}\)) = 12.79
Therefore, the pH of the solution with [\(OH^-\)] = 0.18 M is 12.79.
Learn more about pH here:
https://brainly.com/question/491373
#SPJ11
Related Questions
What is the percentage composition of water?
Answers
Answer:
Water makes up about 70 percent of the human body. The water content in the body of male is more than that in the body of female, and the water content in the body of young people is more than that in the elderly. The water content in the body of newborn is about 70%~75% shui. The amount of water in human tissues is also different: about 10 percent of the water in bone and cartilage; Water in fat accounts for about 20%~35% of total fat; The distribution of water in muscles has reached up to about 70% of the total muscle mass. In the blood plasma, except 6%~8% of plasma proteins, 0.1% of glucose and 0.9% of inorganic salts, the rest are all water, accounting for about 91%~92% of the total plasma.
Explanation:
1.
Sir William Ramsey is one scientist credited with identifying the noble gas argon. Sir Ramsey
separated nitrogen gas from the air and reacted it with an excess of magnesium, producing solid
magnesium nitride. However, a small sample of an unreactive gas remained with a density different
from the density of the nitrogen gas. Sir Ramsey identified the unreactive gas as argon and later
went on to discover neon, krypton, and xenon.
State, in terms of valence electrons, why the noble gases that Sir Ramsey discovered have similar
chemical properties.
Answers
The noble gases have similar chemical properties because they have the same number of valence electrons.
What are noble gases?The noble gases are the gases that are found in the last group of the periodic table. These are the gases that have been said to be unreactive under normal conditions. Thee have been found to react only under stringent conditions as we can see.
Given the fact that the octet rule states that an element that contains eight electrons in its outermost shell would be unreactive and all the noble gases have complete octets hence the noble gases are unreactive.
Learn more about noble gases:https://brainly.com/question/11764545
#SPJ1
When an atom of a Radioactive element emits alpha radiation, an atom of a different element is formed. A different element is formed because the radioactive element has lost...
Answers
Explanation:
its its originality as an atom has to be pure
A different element is formed because the radioactive element has lost its originality as a pure atom when a radioactive element emits alpha radiation.
What is a radioactive element?Some elements of unstable atomic nuclei undergo radioactive decay to form stable nuclei because of the presence of excess nuclear charge inside them are known as radioactive elements.
The stability of nuclei of an element can be estimated by neutron to proton ratio. The atomic number up to Z= 20 is stable nuclei and contains an equal number of protons and neutrons. As the atomic number starts to increase, the repulsion forces between protons increase.
Thus, the neutron-to-proton ratios of stable nuclei increase with increasing atomic numbers. If elements with the atomic number, Z > 83 and n/p > 1.5 and they will be most unstable and radioactive.
For example, Pu-240 emits an alpha particle and gets converted into U-236 which is completely different from the original nuclei.
²⁴⁰Pu₉₄ → ⁴He₂ + ²³⁶U₉₂
Therefore, the radioactive element lost its identity after disintegration.
Learn more about the radioactive element, here:
https://brainly.com/question/23759636
#SPJ2
What does a negative AHf for a molecule mean?
A. Energy was added when the molecule changed phases.
B. Energy was added when the molecule was formed from its
elements.
C. Energy was released when the molecule was formed from its
elements.
O D. Energy was released when the molecule went through a phase
change.
SUBMIT
Answers
Option D I got it right
ANSWER: C (ENERGY WAS RELEASED WHEN THE MOLECULE WAS FORMED FROM ITS ELEMENTS.)
HOPE THE OTHER PERSON IS WRONG!
What are the smallest units an element can be broken down into and still retain its defining properties?
electrons
molecules
nanograms
atoms
Answers
Answer:
The answer to your question is molecules.
Elements o fb the same group have the same chemical properties
Answers
Answer:
The chemical elements are arranged in order of increasing atomic number. The horizontal rows are called periods and the vertical columns are called groups. Elements in the same group have similar chemical properties. This is because they have the same number of outer electrons and the same valency.
is the process of mixing ethanol and water exothermic or endothermic? how do you know (use numeric data to justify)? does this suggest presence of large clusters or small clusters in the mixture?
Answers
The reaction is exothermic as it involves a bond-forming process
when the liquids are mixed temperature of the reaction between the mixture increases.
There are small and large clusters in the mixture
The exothermic reaction involves the formation of new bonds which involves a combination of the bonds to form a new compound in our case water has hydroxy ions that bond with those of ethanol.
Mixing ethanol and water in volumetric glassware reduces the volume and increases the temperature.
The numerical example
Fill two 250 mL graduated cylinders to the line with water and ethanol pure temperature sensor shows both at room temperature. Next, move the temperature probe to an empty 500 mL graduated cylinder and pour the contents of the two smaller cylinders simultaneously and mix well.
The temperature of the mixture will rise by about 8 °C and the volume will decrease to 480 this indicates that the reaction is exothermic as the temperature rises and volume reduces
yes, clusters are present as ethanol falls between the spaces left by water as it has small molecules than that of water.
To learn more about ethanol and water reactions see https://brainly.com/question/1206318?referrer=searchResults:
#SJP4
How do the isotopes hydrogen-1 and hydrogen-2 differ?
Hydrogen-2 has one proton; hydrogen-1 has none.
Hydrogen-2 has one neutron; hydrogen-1 has none.
Hydrogen-2 has one more electron than hydrogen-1.
Hydrogen-2 has two protons; hydrogen-1 has one.
Answers
it is AExplanation because a+w=a
Based off of your solubility chart, which of the following compounds would form a precipitate in water?
a. KCI
c. (NH4)₂S
d. BaSO4
b.NaOH
Answers
The compound that would form a precipitate in water is BaSO4.
option d.
BaSO4 (barium sulfate) would form a precipitate in water because it is classified as an insoluble compound according to most solubility charts. When a compound is considered insoluble, it means that it has a very low solubility in water, resulting in the formation of solid particles or precipitate when dissolved in water.
In the case of BaSO4, it does not readily dissociate into ions in water and remains as solid particles, causing it to precipitate.
On the other hand, a. KCI (potassium chloride), b. NaOH (sodium hydroxide), and c. (NH4)2S (ammonium sulfide) are soluble compounds in water. They dissociate into ions and form homogenous solutions when dissolved in water, without forming a precipitate.
It's worth noting that solubility can vary depending on factors such as temperature and concentration, so it's always important to consult a solubility chart or reference for accurate and up-to-date information on specific compounds.option d.
for such more questions on compound
https://brainly.com/question/29108029
#SPJ8
1. The diagram models the forces acting on a box. 2N 2N- Box - 2 N 5 N Which of the following best describes the motion of the box?
Answers
Answer: The box is moving downward with increasing speed.
Explanation:
The diagram models the forces acting on a box. 2N 2N- Box - 2 N 5 N .The box is moving downward with increasing speed best describes the motion of the box.
What forces act on a box?The normal force acting on the box is equal to and opposite to its weight. This term equals the force of gravity when the box is on a level surface. To calculate the normal force of the box, use our given mass and gravity's acceleration.
When an object interacts with another object, it experiences a push or pull that causes it to change state, either from rest to uniform motion. It is a magnitude and direction vector quantity.
A representation of the forces acting on the object. The object is represented by a dot, and forces are represented by arrows pointing away from the dot. Force diagrams are another name for them.
Thus, The box is moving downward with increasing speed best describes the motion of the box.
To learn more about the force, follow the link;
https://brainly.com/question/13191643
#SPJ6
Imagine our class has discovered a new element and named it Berkmarium. Berkmarium is known to have 3 naturally occurring isotopes. In nature, 70% of the element is Berkmarium-95, 28% is Berkmarium-97, and 2% is Berkmarium-94. Which isotope will the average atomic mass of Berkmarum be closest to, and why? Support your answer with a calculation.
Answers
Answer:
Detail is given below.
Explanation:
Given data;
Abundance of Berkmarium-95 = 70%
Abundance of Berkmarium-97 = 28%
Abundance of Berkmarium-94 = 2%
Average atomic mass closer to which isotope = ?
Solution:
1st of all we will calculate the average atomic mass of Berkmarium.
Average atomic mass = (abundance of 1st isotope × its atomic mass) +(abundance of 2nd isotope × its atomic mass) + (abundance of 3rd isotope × its atomic mass) / 100
Average atomic mass = (70×95)+(28×97)+(2×94) /100
Average atomic mass = 6650 + 2716+ 188 / 100
Average atomic mass= 9554 / 100
Average atomic mass = 95.54 amu
The average atomic mass is closer to the isotope Berkmarium-95 because it is present in abundance as compared to the other two isotope. So this isotope constitute most of the part of Berkmarium.
For the frequency, 4.7 x 10^12 Hz, what is the wavelength?
Answers
How many molecules of water are in a 45 g sample of H2O?
1. 1. 5 x 1024 molecules H2O
2.
1. 3 x 10-21 molecules H20
FREE
3.
3. 7 x 1024 molecules H2O
4.
6. 5 x 1023 molecules H2O.
I need help answering this:/
Answers
The number of molecules of water in a 45 g sample of H2O is approximately 3.7 x 10^24 molecules H2O.
To calculate the number of molecules, we need to use Avogadro's number, which states that there are 6.022 x 10^23 molecules in one mole of a substance. First, we need to determine the number of moles of water in the 45 g sample. The molar mass of water (H2O) is approximately 18 g/mol (2 g/mol for hydrogen and 16 g/mol for oxygen).
Using the formula:
moles = mass / molar mass
moles = 45 g / 18 g/mol
moles = 2.5 mol
Now, to find the number of molecules, we multiply the number of moles by Avogadro's number:
number of molecules = moles * Avogadro's number
number of molecules = 2.5 mol * (6.022 x 10^23 molecules/mol)
number of molecules = 3.7 x 10^24 molecules
Therefore, there are approximately 3.7 x 10^24 molecules of water in a 45 g sample of H2O.
learn more about Avogadro's number here:
https://brainly.com/question/28812626
#SPJ11
You have a solution of chromium(III) ions at a pH of 4 to which you add NaOH until the pH reaches about 7 and a solid powder forms. What is the most likely formula for the solid formed?
Answers
The most likely formula for the solid formed is [Cr(OH)3].
When NaOH is added to a solution of chromium (III) ions at a pH of 4 until the pH reaches 7, a solid powder is formed. The most probable formula of the formed solid is [Cr(OH)3].
Explanation: Chromium (III) ions contain a +3 charge; thus, the addition of NaOH solution produces a precipitate of chromium hydroxide. When a base like NaOH is added to the solution of chromium ions, it reacts with the hydrogen ions (H+) from the acid to produce water and hydroxide ions (OH-).Cr3+ + 3OH- → Cr(OH)3
The formula for the solid formed can be determined by the charge balance of the ions in the chemical reaction.
Chromium (III) hydroxide is formed by the combination of three hydroxide ions (OH-) and one chromium (III) ion (Cr3+). Therefore, the correct formula of the solid formed is [Cr(OH)3].
Finally, it can be concluded that when a solution of chromium(III) ions at a pH of 4 is treated with NaOH until pH reaches about 7, the most likely formula for the solid formed is [Cr(OH)3].
To learn about the chemical reaction here:
https://brainly.com/question/11231920
#SPJ11
Based on the data in Figure 9.13, which of the energy-storing molecules produced during glucose oxidation is expected to carry the highest amount of chemical energy?
- NADH- carbon dioxide- ATP- ETC
Answers
Based on the data in Figure 9.13, the energy-storing molecule produced during glucose oxidation that is expected to carry the highest amount of chemical energy is ATP.
Chemical energy is a type of energy that is created or absorbed when atoms or molecules undergo a chemical reaction. It is the energy kept in the bonds of chemical substances, and it is available when those bonds are broken. The glucose molecule is a great example of a molecule with high chemical energy. When glucose is broken down in the presence of oxygen, most of the energy that was saved in its chemical bonds is moved to ATP molecules.
In the energy-storing molecules generated during glucose oxidation, ATP has the highest amount of chemical energy. It is the most common source of energy utilized by living organisms for cellular processes. The reaction that generates ATP happens in the mitochondria and it is driven by the electron transport chain (ETC). During this process, electrons from NADH and FADH2 travel down a sequence of electron carriers until they reach oxygen, the final electron acceptor. ATP is produced when a gradient of protons is established, and the protons pass through a channel enzyme called ATP synthase, producing ATP.
Therefore, ATP is expected to carry the highest amount of chemical energy.
To learn more about "glucose oxidation", visit: https://brainly.com/question/30871283
#SPJ11
0.670 mol of beryllium nitrate, Be(NO3)2 are required for a specific chemical reaction you want to perform in the lab. What mass of Be(NO3)2 do you need to have precisely 0.670 mol of the compound?
Please Show your work.
Thank you
Answers
The mass of Be(NO₃)₂ needed to have precisely 0.670 mole of the compound is 89.11 grams
0.670 moles of beryllium nitrate, Be(NO₃)₂ is needed for the chemical reaction. The mass of Be(NO₃)₂ needed to have precisely 0.670 moles of the compound can be calculated as follows:
Molecular mass:Molecular mass is a number equal to the sum of the atomic masses of the atoms in a molecule.
molecular mass of Be(NO₃)₂ = 9 + 14(2) + 16(6) = 9 + 28 + 96 = 133 grams per mole
Therefore,
1 mole of Be(NO₃)₂ = 133 grams
0.670 moles = ?
cross multiply
mass required = 0.670 × 133
mass of Be(NO₃)₂ required = 89.11 grams
learn more on mass here:https://brainly.com/question/26177156
Sam did an experiment. He boiled water in a teapot and saw vapor coming out. When he placed a cold plate in contact with the vapor it formed droplets of water.? Can you imagine what was that process and Why did he observe this?
Answers
Answer:
evaporation and condesation
Explanation:
because he was curiuos
what is chemical reaction
Answers
o facilitate ease of dose calculations for cefazolin injection, your department policy
states that the resulting concentration after reconstitution should be 100 mg/mL. The
packaging insert for cefazolin 1-g vial instructs you to add 3.4 mL of sterile water without
bacteriostat, resulting in a reconstituted solution of 250 mg/mL
i. What is the final volume of the reconstituted cefazolin solution?
A 3 mL
B. 4 mL
C. 5 mL
D. 2.5 mL
ii. What is the volume of the cefazolin powder?
A 0.4 mL
B. mL
C. 0.7 mL
D. 0.6 mL
iii. What is the final volume of the 100mg/mL cefazolin solution?
A. 6 mL
B. 8 mL
C. 7 mL
D. 10 mL
Answers
The final volume of the reconstituted cefazolin solution is 4 mL. The volume of the cefazolin powder is 0.6 mL. The final volume of the 100 mg/mL cefazolin solution is 10 mL.
The packaging insert instructs to add 3.4 mL of sterile water without bacteriostat to the 1-g vial of cefazolin. This results in a reconstituted solution with a concentration of 250 mg/mL.
To find the final volume, we can set up the equation:
Concentration of reconstituted solution = Amount of drug / Final volume
Using the given concentration (250 mg/mL) and the amount of drug (1 g = 1000 mg), we can rearrange the equation to find the final volume:
250 mg/mL = 1000 mg / Final volume
Solving for the final volume:
Final volume = 1000 mg / 250 mg/mL = 4 mL
Therefore, the final volume of the reconstituted cefazolin solution is 4 mL.
To find the volume of the cefazolin powder, we need to subtract the volume of sterile water added from the final volume of the reconstituted solution.
Given that 3.4 mL of sterile water is added to the vial, and the final volume of the reconstituted solution is 4 mL, we can calculate the volume of the cefazolin powder as follows:
Volume of cefazolin powder = Final volume - Volume of sterile water added
Volume of cefazolin powder = 4 mL - 3.4 mL = 0.6 mL
Therefore, the volume of the cefazolin powder is 0.6 mL.
To determine the final volume of the 100 mg/mL cefazolin solution, we can use the concentration and the amount of drug.
We are given that the resulting concentration after reconstitution should be 100 mg/mL. Considering the amount of drug is 1 g (1000 mg), we can set up the following equation:
Concentration of reconstituted solution = Amount of drug / Final volume
Using the given concentration (100 mg/mL) and the amount of drug (1000 mg), we can rearrange the equation to find the final volume:
100 mg/mL = 1000 mg / Final volume
Solving for the final volume:
Final volume = 1000 mg / 100 mg/mL
Final volume = 10 mL
Therefore, the final volume of the 100 mg/mL cefazolin solution is 10 mL.
To know more about cefazolin visit:
https://brainly.com/question/8549780
#SPJ11
a 14.71 g sample of nabr contains 22.34% na by mass. how many grams of sodium does a 6.45 g sample of sodium bromide contain?
Answers
1.44 grams of sodium does a 6.45 g sample of sodium bromide contain.
What is sodium bromide?
The chemical compound sodium bromide has the formula NaBr. It is synthetic. It resembles sodium chloride and is a highly volatile, white, crystalline substance. The bromide radical is frequently utilised with this salt. In the chemistry and pharmaceutical industries, this salt has several uses. It cannot naturally occur in the rocks or naturally occurring minerals due to its solubility. Halides, chlorides, and iodides are a few of the chemical substances that are recovered from the ocean water.
22.34% of the mass of Na will be present in any sample of NaBr.
Thus, the 14.71 g is unimportant.
22.34 % of 6.45 = 0.2234 x 6.45 = 1.44093g
Cite your response for 3 significant numbers, which is 1.44 g, since 22.34 has 4 significant figures and 6.45 has 3.
To learn more about sodium bromide visit;
https://brainly.com/question/15409724
#SPJ4
Please help due today
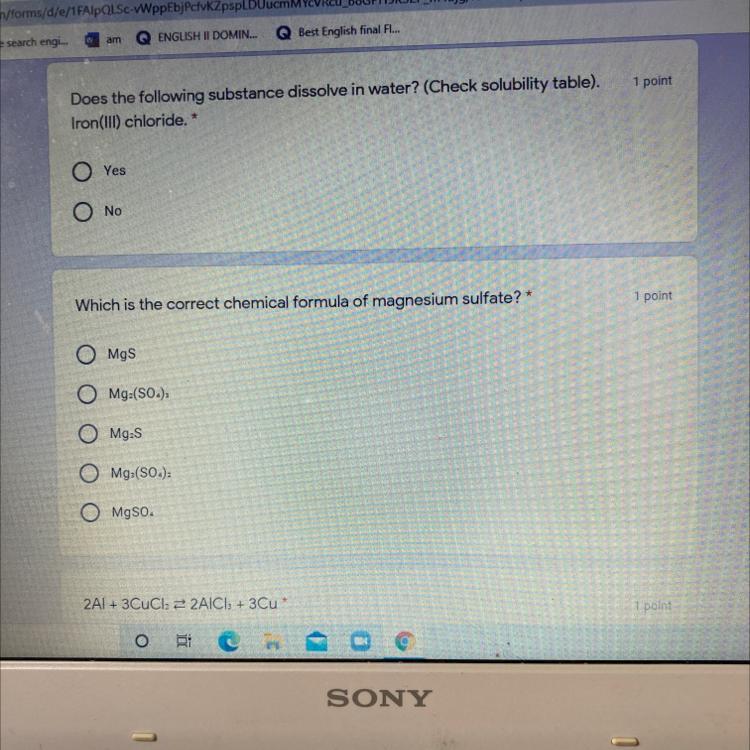
Answers
Answer:
the answer for the magnesium sulphate is MgSO4
Answer:
For the first one it's no.
On your Second question it's MgS.
Determine the number of oxygen atoms present in 30.2 g carbon dioxide.
____ atoms
Answers
Answer:42 atoms at least
42
Explanation:
1234567912345678912345678912345678
Conversion of wintergreen oil to salicylic acid is this type of reaction
Answers
The conversion of wintergreen oil (methyl salicylate) to salicylic acid is an example of a hydrolysis reaction.
Hydrolysis is a chemical process in which a molecule is cleaved into two parts by the addition of a water molecule. In this specific case, methyl salicylate reacts with water under acidic or basic conditions to produce salicylic acid and methanol.
The presence of a catalyst, such as a strong acid like sulfuric acid (H₂SO₄) or a base like sodium hydroxide (NaOH), helps to speed up the reaction. In an acidic environment, the ester bond in methyl salicylate is protonated, making it more susceptible to nucleophilic attack by water. In a basic environment, the hydroxide ion (OH-) acts as a nucleophile and attacks the ester bond.
The products of this hydrolysis reaction are salicylic acid, a widely used compound in the pharmaceutical industry for its anti-inflammatory and analgesic properties, and methanol, a simple alcohol. This reaction is particularly relevant because it demonstrates the transformation of naturally occurring compounds, like wintergreen oil, into valuable industrial chemicals, such as salicylic acid, which is used as a key ingredient in many medications and cosmetic products.
Learn more about Hydrolysis here: https://brainly.com/question/1403345
#SPJ11
for the following reaction, k < 1. classify each of the reactants and products based on their strength as bronsted-lowry acids or bases. c6h5oh c6h15o3n c6h15o3nh c6h5o- c6h5o- c6h5oh c6h15o3nh c6h15o3n 1) stronger bronsted-lowry acid 2) weaker bronsted-lowry acid 3) stronger bronsted-lowry base 4) weaker bronsted-lowry base
Answers
For the given reaction, we can classify the reactants and products as follows:
1) Stronger Brønsted-Lowry acid: C₆H₅OH (phenol)
2) Weaker Brønsted-Lowry acid: C₆H₅O⁻ (phenolate ion)
3) Stronger Brønsted-Lowry base: C₆H₁₅O₃NH (protonated triethanolamine)
4) Weaker Brønsted-Lowry base: C₆H₁₅O₃N (triethanolamine)
It is important to note that the strength of a Bronsted-Lowry acid or base is related to its ability to donate or accept a proton (H+ ion) in a chemical reaction. A stronger acid is one that can easily donate a proton, while a stronger base is one that can easily accept a proton.
In this case, C₆H₅OH is a stronger acid than C₆H₁₅O₃N because it has a more acidic hydrogen ion. Similarly, C₆H₁₅O₃NH is a stronger base than C6H5O- because it has a greater ability to accept a proton.
Learn more about acid-base at
https://brainly.com/question/32276007
#SPJ11
Which of the following elements has the highest electronegativity?
*potassium
*lithium
*chlorine
*oxygen
Answers
How many moles are there in 134.76 grams of potassium nitrate?
Answers
To calculate the number of moles of potassium nitrate in 134.76 grams, we can use the following formula:
Number of moles = Mass / Molar mass
Number of moles = 134.76 g / 101.1 g/mol
Number of moles = 1.33 mol
Therefore, there are 1.33 moles of potassium nitrate in 134.76 grams of the substance.
Volcanoes can be destructive LOCALLY causing all of the following immediate effects EXCEPT:
O New growth in forests
O Personal damage
O Lack of breathable air
O Disruption of clean water
O Death
Answers
Answer:
New growth of trees is an exception
Explanation:
when volcanoes erupt, they release hot magma that is destructive to the environment causing personal damage, lack of breathable air and death.
heat produce cannot in any way help in growth of trees
HELP PLEASEE 100 POINTS
The Quiver tree grows in Southern Africa. Which of the following plant adaptations is likely to prevent these trees from dying out due to rising desert temperatures?
O Releasing a black powder onto their trunk to absorb more heat from sunlight
O Shifting their growing range towards the equator
Ability to store water in leafy structures to prevent excess evaporation
O Limiting seed dispersal to nearby locations
PLEASE HELP 100 POINTS !!
Answers
Answer:
Shifting their growing range towards the equator
Explanation:
Maybe will be like that
Answer:
Ability to store water in leafy structures to prevent excess evaporation.
Explanation:
The reason why plants die in hot temperature is the excess evaporation of water, so to prevent the excess evaporation of water, the plants get adaptive to store water in their leaves.
Hope it helps.
What is produced at the anode in the electrolysis of molten CuF2? O A. F2 O B. H2 O C. cu D.02
Answers
The produced at the anode in the electrolysis of molten CuF2 is A. F₂.
During electrolysis, CuF₂ will separate into Cu₂⁺ cations and F⁻anions. Cu₂⁺ will be reduced to copper metal, which will deposit on the cathode, whereas F⁻ anions will be oxidized to fluorine gas on the anode in the electrolysis of molten CuF₂. Hence, the answer is option A. Fluorine gas (F₂) is generated at the anode in the electrolysis of molten CuF₂.
Therefore, during the electrolysis of molten CuF₂, Cu₂⁺ is reduced to copper metal, which deposits on the cathode, and F⁻ anions are oxidized to fluorine gas on the anode, which is produced at the anode. The chemical reactions taking place during electrolysis of CuF₂ are given below: At the cathode, Cu₂⁺ cations get reduced to copper metal. Cu₂⁺ + 2e⁻ ⟶ Cu. At the anode, F⁻ anions get oxidized to fluorine gas. 2F⁻ ⟶ F₂ + 2e⁻. Therefore, option A is correct.
Learn more about electrolysis at:
https://brainly.com/question/12994141
#SPJ11
Question 20 of 30
What does the absorption spectrum of an atom show?
A. The wavelengths of light that an atom gives off when electrons
fall back to lower energy levels
OB. The temperature of the phase transitions of the element at
different pressures
O C. The amount of energy that is absorbed as the element changes
phase
O D. The wavelengths of light that cause the electrons in the atom to
move to higher energy levels
Answers
The wavelengths of light that cause the electrons in the atom to move to higher energy levels. Option D
What is the atomic spectrum?The wavelengths of light that are absorbed by an atom as its electrons move from lower to higher energy levels are shown by the atom's absorption spectrum. When exposed to light, atoms are able to absorb a range of wavelengths depending on the energy differences between the levels of their electrons.
The encouragement of electrons to higher energy levels within the atom results from this absorption. The absorption spectrum, which is often represented graphically as dark lines or bands on a continuous spectrum, is a representation of the wavelengths of light that are absorbed by the atom.
Learn more about atomic spectrum:https://brainly.com/question/28007345
#SPJ1