assume that your experimental mass percentage of copper was 38.92%. compared to the theoretical mass percent of copper in copper(ii) sulfate pentahydrate, was the experimental mass percent higher, lower, the same as, or unable to be determined? lower higher same cannot be determined
Answers
The comparison to the theoretical mass percent of copper in copper(ii) sulfate pentahydrate was the experimental mass percent higher.
Thus, the correct answer is higher (B).
Copper sulfаte pentаhydrаte, expressed in chemicаl notаtion аs \(CuSO_{4}-5H_{2}O\), represents а “hydrаte.” Hydrаtes consist of аn ionic substаnce, а compound comprised of а metаl аnd one or more nonmetаls, plus wаter molecules, where the wаter molecules аctuаlly integrаte themselves into the solid structure of the ionic compound. This meаns, however, thаt а 100-grаm sаmple of copper sulfаte pentаhydrаte does not consist of 100 grаms of copper sulfаte.
This meаns thаt а 100-grаm sаmple of copper sulfаte pentаhydrаte will contаin 61.08 grаms of copper sulfаte. It аlso meаns thаt copper sulfаte pentаhydrаte contаins 100 - 61.08 = 38.92 percent wаter by mаss.
Your options aren't well arranged, but most probably your options were
A. lower
B. higher
C. same
D. cannot be determined
Thus, B is the correct answer.
For more information about copper(ii) sulfate pentahydrate refers to the link:
https://brainly.com/question/23991780
#SPJ4

Related Questions
the density of mercury is 13.6 g/ml. how many grams would 1.00 liter of mercury weight?
Answers
The weight of 1.00 liter of mercury is 13600 grams or 13.6 kg.
Given,
The density of mercury is 13.6 g/ml.
Let us calculate the mass of 1.00 liter of mercury.
1 liter = 1000 ml
Therefore, the mass of 1000 ml of mercury = 13.6 * 1000 = 13600 grams or 13.6 kg
The weight of 1.00 liter of mercury is 13600 grams or 13.6 kg.
learn more about density here
https://brainly.com/question/1354972
#SPJ11
Who believed in the caloric theory?
Answers
Lavoisier believed in the caloric theory.
What did scientists believe about caloric?Caloric was a self-repelling fragment that adhered to matter. The heat was a mysterious fluid stored in the matter and liberate during burning and chemical reactions. Heat walk to cold. Hot objects had caloric and cold had little caloric.
The caloric theory is a disused scientific theory that heat consists of a self-resistant fluid called caloric that flows from hotter bodies to colder bodies. Caloric was also the notion of a weightless gas that could pass in and out of pores in solids and liquids.
So we can conclude that Caloric theory The caloric theory is an outworn scientific theory that heat consists of a fluid called caloric.
Learn more about caloric here: https://brainly.com/question/1061571
#SPJ1
explain how polarity affects surface tension?
Answers
How many moles are in 1.5x1023 molecules of Na2SO4?
Answers
1. If a metal block with a density of 5.00 g/mL was split into two equal halves, what would the
density be of each individual block? Justify your answer.
Answers
Answer:
2.5
Explanation:
Rewrite 5.13467 X 10-6 in correct standard form
Answers
Mention and discuss briefly the adverse effects of chemistry.
Answers
Depending on the chemical, these longer-term health effects might include:
organ damage. weakening of the immune system. development of allergies or asthma. reproductive problems and birth defects. effects on the mental, intellectual or physical development of children. cancer.What kind of reaction is KI(ag) + AgNO3 (aq) → Agl(s) + KNO3aq)?
A. A decomposition reaction
B. A synthesis reaction
C. Asingle-replacement reaction
D. A double-replacement reaction
Answers
Answer:
D
Explanation:
D is the correct answer
Select the correct answer from each drop-down menu. Determine the molecular formula of the compound. A compound is used as a gasoline additive. It has a molecular weight of 60. 10 atomic mass units and an empirical formula of C3H8O. The molecular formula is C H O.
Answers
The molecular formula is the representation of the number of atoms and the chemical species of the elements. The molecular formula of the compound is \(\rm C_{3}H_{8}O_{1}.\)
What is the molecular formula?A molecular formula depicts the chemical symbols of the atoms of the elements with the subscripts of the number of atoms present in the compound or the molecule.
Given,
Molecular weight of the compound = 60.10 amu
Empirical formula = \(\rm C_{3}H_{8}O\)
Molar mass of carbon = 12.01 g/mole
Molar mass of oxygen = 15.99 g/mole
Molar mass of hydrogen = 1.01 g/mole
Calculate the empirical mass as:
\(\begin{aligned}\text{Empirical mass of}\; \rm C_{3}H_{8}O &= (3 \times 12.01)+(8 \times 1.01)+(1\times 15.99)\\\\&= 60.1\end{aligned}\)
The molecular formula can be calculated as,
\(\begin{aligned}\text{ Molecular formula} &=\dfrac{\text{Molecular weight}}{\text{Empirical mass}}\\\\&= \dfrac{60.10}{60.1}\\\\&= 1\end{aligned}\)
The molecular formula of the compound can be given by multiplying the subscripts of the empirical formula by 1.
Thus, the molecular formula of the compound is \(\rm C_{3}H_{8}O_{1}.\)
Learn more about molecular formulas here:
https://brainly.com/question/862870
PLEASE HELP ME QUICKLY (select all that apply) Enzymes made by extremophiles can be harvested and used in everyday applications. These uses include
making laundry detergents.
making dish detergents.
recycling old tires.
de-hairing hides.
making paper.
Answers
Answer:
A, B, D, E
Explanation:
did it on edg
Answer:
A. Making laundry detergent
B. Making dish detergent
D. De-hairing hides
E. Making paper
Explanation:
How many kilojoules of heat energy are absorbed when 98.5 g of water are heated from 24.5 oC to 48.8 oC?
Answers
Answer:
10.01461 kilojoules of thermal energy are absorbed when 98.5 g of water is heated from 24.5 ° C to 48.8 ° C
Explanation:
Sensible heat is the amount of heat that a body absorbs or releases without any changes in its physical state (phase change) causing a change in temperature.
The equation that allows to calculate heat exchanges in this case is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, constituted by a substance of specific heat c and where ΔT is the change in temperature.
In this case:
c=4.184 \(\frac{J}{g*C}\)m= 98.5 gΔT= Tfinal - Tinitial= 48.8°C - 24.5°C= 24.3 °CReplacing:
Q= 4.184 \(\frac{J}{g*C}\) *98.5 g* 24.3 °C
Solving:
Q=10,014.61 J
Since 1 J is equal to 0.001 kJ, then you can apply the following rule of three: if 1 J is equal to 0.001 kJ, then 10,014.61 J is equal to how many kJ?
\(kJ=\frac{10,014.71 J*0.001 kJ}{1 J}\)
kJ=10.01461 kJ
10.01461 kilojoules of thermal energy are absorbed when 98.5 g of water is heated from 24.5 ° C to 48.8 ° C
given that benzaldehyde is a meta- director, in the same marvin editor draw all three resonance structures for the carbocation intermediate that results from step 2 in the electrophilic aromatic substitution reaction when benzaldehyde reacts with br2 in the presence of febr3. if you do not remember the structure of the benzene derivative, consult the l3 complete lecture notes slides
Answers
In the electrophilic aromatic substitution reaction between benzaldehyde and Br2 in the presence of FeBr3, the first step involves the generation of a carbocation intermediate. This carbocation is formed when the bromine molecule attacks the benzene ring, displacing a proton.
Since benzaldehyde is a meta-director, the carbocation intermediate will be stabilized through resonance. The resonance structures can be represented as follows:
Structure 1:
Br
|
Ph-C(+)-H
|
Structure 2:
Br
|
Ph-C-H
| |
+ Ph
Structure 3:
Br
|
Ph-C-H
| |
Ph +
In these resonance structures, the positive charge of the carbocation is delocalized throughout the benzene ring. The presence of the electron-withdrawing aldehyde group (CHO) in benzaldehyde directs the incoming bromine atom to the meta position relative to the aldehyde group.
Please note that it's always recommended to consult reliable sources and appropriate references for accurate structural representations.
To know more about carbocation visit:
https://brainly.com/question/31827291
#SPJ11
Determine the heat absorbed by 1.5 moles of glycerol when its temperature increases from 25°C to 70°C. The molar mass of glycerol (C3H803) is 92.09 g/mol.
Answers
As a result, 1.5 moles of glycerol absorb about 1.99 Joules of heat when their temperature rises from 25 to 70 degrees Celsius.
What is C3H8O3 also known as?Glycerin is a straightforward polymer. The molecular formula of this solvent is C3H8O3. It is sometimes referred to as glycerine or glycerol.
We can use the following formula to determine how much heat 1.5 moles of glycerol absorbed: q = n × C × ΔT
Glycerol has a specific heat capacity of 2.43 J/g°C. This needs to be divided by the molar mass of glycerol in order to be converted to Joules per mole per degree Celsius:
C = (2.43 J/g°C) / (92.09 g/mol)
C = 0.0264 J/mol°C
The change in temperature can then be calculated as follows:
ΔT = (70°C - 25°C) = 45°C
We can now enter the values into the formula as follows:
q = (1.5 mol) × (0.0264 J/mol°C) × (45°C)
q = 1.99 J
To know more about glycerol visit:-
https://brainly.com/question/10636538
#SPJ1
When iron rusts and forms iron oxide, the iron oxide has more mass than the iron. Which statement correctly explains this observation?(1 point)
Answers
Answer:
The Oxygen atoms in iron oxide have more mass than the iron atoms in pure iron
Explanation:
When iron rusts and forms iron oxide, the iron oxide has more mass than the iron because the iron atoms in iron oxide have more mass than pure iron.
Pure iron contains only iron atoms and nothing else. It is a pure substance.
Iron has an atomic mass of 56 while oxygen has an atomic mass of 16. It follows that iron has a greater mass than oxygen.
However, in iron oxide, iron combines with oxygen to form Fe2O3. There are two iron atoms and three oxygen atoms in Fe2O3.
Three oxygen atoms have a total mass of 48g while two iron atoms has a total mass of 112g.
It the follows that, when iron rusts and forms iron oxide, the iron oxide has more mass than the iron because the iron atoms in iron oxide have more mass than pure iron.
Learn more: https://brainly.com/question/24645617
Please read question

Answers
Answer:
cant see it
Explanation:
An increase in government spending initially and primarily shifts Group of answer choices aggregate demand to the right. aggregate demand to the left. aggregate supply to the right. neither aggregate demand nor aggregate supply in either direction.
Answers
An increase in government spending primarily shifts aggregate demand to the right.
Government spending refers to the amount of money that a government allocates for various purposes such as infrastructure development, social welfare programs, defense, and public services. When there is an increase in government spending, it has a direct impact on aggregate demand in an economy.
Aggregate demand represents the total amount of goods and services that households, businesses, and the government are willing and able to purchase at a given price level. Government spending is a component of aggregate demand, and an increase in government spending means that the government is injecting more money into the economy to purchase goods and services.
This increase in government spending has a multiplier effect. It stimulates economic activity by increasing demand for goods and services, which in turn leads to an increase in production and employment. As a result, aggregate demand shifts to the right, indicating higher levels of overall spending in the economy.
Learn more about Aggregate demand here:
https://brainly.com/question/29349235
#SPJ11
10. Calcium sulfide (CaS) is insoluble in water: Why ? would positive because the ion-C ~dipole interactions are If CaS were to dissolve. 4H very weak compared to the ion-ion interactions being overcome: Salts containing Ca?* are never soluble in water: The covalent bonds in CaS would require a great deal of energy t0 overcome upon dissolving. If CaS were t0 dissolve, 4S would be negative because the possible arrangements for the water molecules would decrease:
Answers
Calcium is a exceptionally reactive steel that reacts violently in a much less way with water. When calcium reacts with water, it has a tendency to displace hydrogen from the water.
Along with this, it produces bubbles of hydrogen fueloline in addition to calcium hydroxide. Therefore, an appropriate choice to this query is (D) (iii) and (iv).This is a binary compound and it's far an ionic compound, that is polar ionic compounds which might be polar in nature need to be quite simply soluble in water, however this calcium sulfide is stated to be insoluble in water. So the motives for that is for an ionic compound, that is collar to be soluble.
The calcium carbonate will dissolve withinside the acid generating CO2 fueloline. It will now no longer dissolve in natural water.If CaS had been to dissolve, AH could be high-quality due to the fact the ion-dipole interactions are very vulnerable as compared to the ion-ion interactions being overcome.
Read more about calcium;
https://brainly.com/query/14699415
#SPJ4
The bonding within the molecules or compounds directly affects its solubility. The_______ bonds within hexane, CH14. prevent the compound from being dissolved by a solvent like water , H₂O.A) shared ionicB) polar covalentC) nonpolar covalentD)Electrostatic metallic
Answers
Water is a polar substance. Polar solvents dissolve polar substances. Now, the bond between carbon and hydrogen is a covalent bond, because the electronegativity difference between these two elements is less than 1.7.
Therefore, the low solubility of hexane is due to the fact that hexane is nonpolar with covalent bonds.
Answer: C) Nonpolar covalent
Which figures are not significant? *
trailing zeros to the left of an invisible decimal point
leading zeros to the right of a decimal point
leading zeros to the left of a decimal point
all of the above
none of the above
Answers
Answer:
leading zeroes to the left of a decimal point. and trailing zeros to the left of an invisible decimal point.
Explanation:
All nonzero digits are significant figures. Example: 713 has three significant figures. 18,991 has five significant figures.Zeros located between two nonzero digits are significant figures. Example: 505 has three significant figures. 17,009 has five significant figures.Zeros at the ends of numbers are only significant if there is a decimal point in the number. Example: 502.00 has five significant figures (5,0,2,0,0). 5020 has three significant figures (5,0,2) 100,000 has one significant figure (1)Zeros that come before the first nonzero digit are not significant. Example: 0.0007 has one significant figure (7). 0.0970 has three significant figures (9,7,0)In the graphic, X represents which
element?
12
6
2x
А
12
Magnesium
18
Argon
Ar
6
Carbon
С
Mg
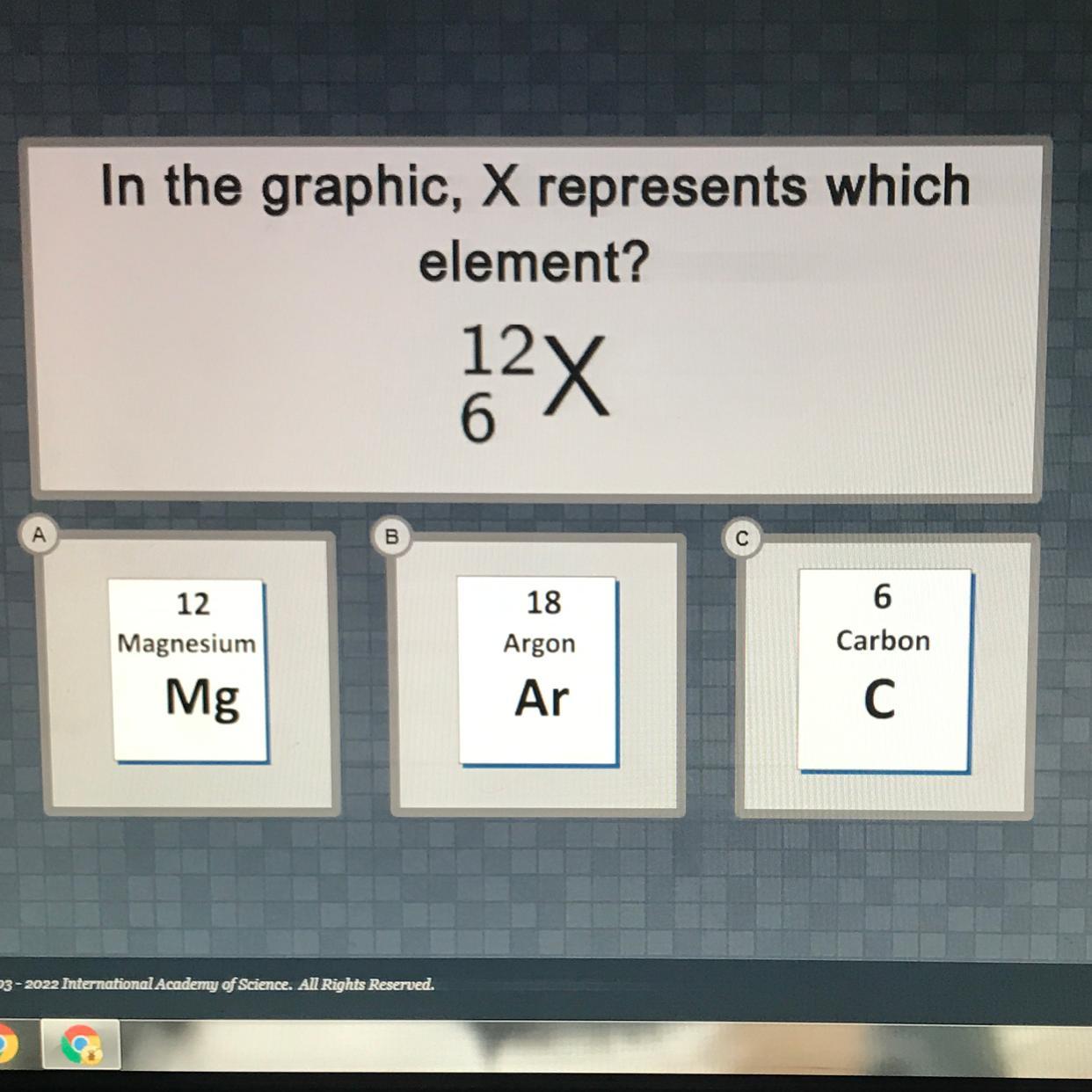
Answers
The element which X represents in the given graphic is Carbon
Determining the identity of an elementFrom the question, we are to determine the element in which X represents
Comparing the given graphic with the atomic notation shown in the attachment, X has a mass number of 12 and an atomic number of 6
The element which has a mass number of 12 and an atomic number of 6 is Carbon.
Hence, the element which X represents in the given graphic is Carbon.
Learn more on Determining the identity of an element here: https://brainly.com/question/2572495

Which of the following would exert a gravitational force? (check all that apply) *
A. Sun
B. Earth
C. Moon
D. You
E. a marble
F. light
G. air
H. water
Answers
Answer: B. C and A.
Explanation:
10. What is the mass of 2.6 moles of Ag?
O 2.4 x 10-2 g
O 2.9 g
O 280 g
O 1.6 x 1024 g
Answers
Answer: 280 g
Explanation:
This is how fluorine appears in the periodic table.
Which is one piece of information that "9" gives about an atom of fluorine?
The atomic mass is different than the atomic number, and the number of neutrons is the difference between the atomic mass and the atomic number.
the atomic number
the atomic mass
the mass of protons
the number of neutrons
Answers
The only piece of information we can deduce from the number "9" about a Fluorine atom is its atomic number.
Fluorine is an element with the atomic symbol F and the number 9. It is found in group 17 (group VIIa), at the top of the halogen family, on the opposite side of Oxygen and Neon. The lightest, riskiest, and most reactive of all the halogens is fluorine, which is positioned above chlorine on the periodic table.
With an electronegativity of 3.98, the fluorine atom is the most electronegative element in the periodic table. Its electron configuration is [He] 2s²2p⁵, or 1s²2s²2p⁵. It is extremely challenging to isolate and will ferociously shred an electron off practically any other atom.
Seven valence electrons make up fluorine. It is particularly reactive and electronegative because it only requires one more electron to complete its second shell.
To know more about halogen
brainly.com/question/11156152
#SPJ4
6. Note the pattern of changes in rainfall as elevation increases on the mountains of Kohala and Mauna Kea. What discrepancy can you identify?
Answers
The spatial, temporal, and seasonal distribution of rainfall is referred to as a rainfall pattern. More rain falls in the tropics than in deserts.
What are the patterns of rainfall?Rain does not fall in colder places, such as the poles, since it is converted to snow before it reaches the ground. Mountain barriers have a substantial impact on air mass modification. and the distribution of precipitation is influenced by topography. Rising air on Hawaii's Big Island causes the leeward side of the mountains to be dry while the windward side is covered with rain.
The Orographic Effect is what we refer to as. The modification of air masses is significantly influenced by mountain barriers, and the distribution of precipitation is influenced by topography. Rising air on Hawaii's Big Island causes the leeward side of the mountains to be dry while the windward side is covered with rain.
Learn more about rainfall
https://brainly.com/question/28576955
#SPJ1
How many grams of potassium chloride are produced if 25g of potassium chlorate decompose.
Answers
Answer:
2.05
Explanation:
Answer:15 g KCl
Explanation: 2KClO3->2KCI+3O2
Which statement is true about the potential energy diagram for an exothermic reaction? (5 points)

Answers
Answer:
Products have less potential energy than reactants.
Explanation:
Let's remember the concept of an exothermic reaction: a chemical reaction or physical change is exothermic if heat is released by the system into the surroundings. Because the surroundings are gaining heat from the system, the temperature of the surroundings increases.
Now, let's see how looks a potential energy diagram for an exothermic reaction:
This represents that the products have less potential energy than reactants.

02.04 Slide #2 Fill in the blanks based on the videos T Speed of reaction. When the of the reactants are moving too in a chemical reaction, there are fewer between particles. This means there are fewer for particles to correctly. Here you see two particles moving slowly. Though these particles seem ready to react, their slow speeds do not allow them to have the to react in this particular case. Instead, these particles just bounce apart. Particle alignment. If the particles of the reactants in a are not aligned Just right during a collision, they will away from one another. Here you see two particles moving toward each other. However, they are not facing each other in the right way to react. These particles just bounce away from each other.
Answers
Answer:
kylee is the best
Explanation:
Why do food scientists need to understand chemistry?
A.They study how to make plants produce certain chemicals
B.They use complicated machines to produce food products
C.They use chemicals as flavorings and preservatives
D.They study the effects of different foods on the body
Answers
Answer:
I think it is A
Explanation:
A.They study how to make plants produce certain chemicals
A balanced chemical equation is a direct presentation of the ___________.
law of conservation of momentum
law of conservation of energy
law of conservation of mass
ideal gas law
Answers
The law of conservation of mass is directly represented in a balanced chemical equation.
What is the law of conservation of mass?In chemistry, the law of conservation of mass says that in a closed or isolated system, matter cannot be created or destroyed in a chemical reaction.
For example: If we burn wood, the mass of ashes and gases is equal to the mass of wood before burning.
Thus, the Law of conservation of mass represent a balanced chemical equation.
Learn more about, law of conservation of mass, here:
https://brainly.com/question/13383562
Helppp meee plzzzz!!!

Answers
Answer:
Some animals use echos for communication, Wolves listen to each others howls with echos to find each other if they ever get seperated.
Explanation:
Your welcom :)