an unknown metal crystallizes in a primitive cubic unit cell. the length of the unit cell edge is 285 pm. the radius of the unknown atom is ________ pm.
Answers
The radius of the unknown metal atom is approximately 142.5 pm.
Based on the information provided, we can calculate the radius of the unknown atom in the primitive cubic unit cell.
In a primitive cubic unit cell, there is one atom at each corner, and the atoms touch along the edge.
The length of the edge is equal to twice the atomic radius.
Edge length = 2 x atomic radius
Given the edge length is 285 pm, we can solve for the atomic radius:
Atomic radius = Edge length / 2
Atomic radius = 285 pm / 2
Atomic radius = 142.5 pm
So, the radius of the unknown atom is 142.5 pm.
To know something about the radius of a metal, click below.
https://brainly.com/question/29574101
#SPJ11
Related Questions
Ion-dipole interactions can occur between any ion and any molecule with a dipole. Identify all of the following pairs of species that can interact via ion-dipole forces. Select all that apply.
a. H2O and CH3OH
b. Li+ and ClO2−
c. NO3− and CH4
d. Li+ and H2O
e. CH3OH and Na+
f. Cs+ and CH3CH2Cl
Answers
Ion-dipole interactions occur between an ion and a molecule with a dipole. These forces are significant in solutions and play a crucial role in various chemical processes. Based on this information, the pairs that can interact via ion-dipole forces are:
b. Li+ and ClO2−
d. Li+ and H2O
e. CH3OH and Na+
f. Cs+ and CH3CH2Cl
These pairs include an ion (Li+, ClO2−, Na+, or Cs+) and a molecule with a dipole (H2O, CH3OH, or CH3CH2Cl).
Ion-dipole interactions occur when an ion interacts with a molecule that has a dipole. In the given pairs, the following species can interact via ion-dipole forces:
a. H2O and CH3OH - Both molecules have a dipole, so they can interact via ion-dipole forces.
b. Li+ and ClO2− - Both ions do not have a dipole, so they cannot interact via ion-dipole forces.
c. NO3− and CH4 - CH4 does not have a dipole, so it cannot interact with NO3− via ion-dipole forces.
d. Li+ and H2O - H2O has a dipole, so it can interact with Li+ via ion-dipole forces.
e. CH3OH and Na+ - CH3OH has a dipole, so it can interact with Na+ via ion-dipole forces.
f. Cs+ and CH3CH2Cl - CH3CH2Cl has a dipole, so it can interact with Cs+ via ion-dipole forces.
Ion-dipole interactions are attractive forces that occur between an ion and a molecule that has a dipole. The ion interacts with the partial charges on the dipole of the molecule, resulting in a stable complex. The strength of the interaction depends on the magnitude of the ion's charge and the dipole moment of the molecule. Molecules with higher dipole moments will have stronger ion-dipole interactions. In the given pairs, only those species that have a dipole can interact with ions via ion-dipole forces. These interactions play a crucial role in many biological, chemical, and physical processes, including solubility, hydration, and reactions in solution.
To know more about ion-dipole forces visit:
https://brainly.com/question/30593470
#SPJ11
3. (5 points) What is the value of 0 for a mixture of polymer A (Sp = 9.0 (cal/cm3)0.5) and the solvent B (85 = 7.5 (cal/cm3)0.5)? Show your work for full credits. NOTE: Assume Vs/RT = 1/6 and a fudge factor of 0.34 in corresponding units. A. 0.375 B. 0.5 C. 0.715 D. 0.035
Answers
The value of θ for the mixture of polymer A and solvent B is 0.375.
So, the correct answer is A.
To determine the value of θ for the mixture of polymer A and solvent B, we need to use the Hildebrand solubility parameter equation:
θ = (Sp_A - Sp_B)² * Vs/RT * fudge_factor
Given values:
Sp_A = 9.0 (cal/cm³)⁰·⁵
Sp_B = 7.5 (cal/cm³)⁰·⁵ Vs/RT = 1/6
fudge_factor = 0.34
Now, let's calculate θ:
θ = ((9.0 - 7.5)²* (1/6) * 0.34
θ = (1.5²) * (1/6) * 0.34
θ = 2.25 * (1/6) * 0.34
θ = 0.375
Hence, the answer of the question is A.
Learn more about mixture of polymer at
https://brainly.com/question/32063861
#SPJ11
Scientific Method
Vocabulary
To notice similarities or differences
Answers
Answer:
The first step would be searching for your scientific vocabulary the second step is to write it down and then that would be your answer.
Explanation:
Hope this helps!
a a reaction orrcurs in a calorimeter that contains 2300g of water at 30c. the reaction releases 9.66 *10^3 j of heat. if the specific heat capacity of water is 4.184 j*g*c what is the final temperature of the water
Answers
The final temperature of the water is 33.02°C.
Here's the solution:
Initial temperature of water (T1) = 30°C
Mass of water (m) = 2300g
Specific heat capacity of water (c) = 4.184 J/g°C
Heat released by the reaction (q) = 9.66 * 10^3 J
Final temperature of water (T2) = (T1 + q/mc)
= (30°C + 9.66 * 10^3 J / 2300g * 4.184 J/g°C)
= 33.02°C
The heat released by the reaction is absorbed by the water, causing the temperature of the water to increase.
The final temperature of the water is calculated by adding the heat released by the reaction to the initial temperature of the water and dividing by the mass of the water and the specific heat capacity of water.
Thus, the final temperature of the water is 33.02°C.
To learn more about specific heat capacity :
https://brainly.com/question/29792498
#SPJ11
If 47.0mL of 4.00 M H2SO4 diluted to 0.160 L, what would be the molarity
Answers
The molarity of the diluted H2SO4 solution is 1.18 M.
What is molarity?Molarity is a measure of the concentration of a solution and is defined as the number of moles of solute dissolved in one liter of solution. It is usually denoted by the symbol M and has units of moles per liter (mol/L).
Molarity can be determined using the following formula:
Molarity (M) = moles of solute (n) divided by volume of solution (V)
where n is the number of moles of solute dissolved in the solution, and V is the volume of the solution in liters.
To find the molarity of the diluted H2SO4 solution, we can use the formula:
M1V1 = M2V2
where M1 and V1 are the initial concentration and volume of the solution, and M2 and V2 are the final concentration and volume of the solution.
In this problem, we have:
M1 = 4.00 M (the initial concentration of H2SO4)
V1 = 47.0 mL = 0.0470 L (the initial volume of the solution)
V2 = 0.160 L (the final volume of the solution, after dilution)
M2 = ? (the final concentration of the solution, which we want to find)
Swapping these values into the formula, we get:
M1V1 = M2V2
4.00 M × 0.0470 L = M2 × 0.160 L
Solving for M2, we get:
M2 = (4.00 M × 0.0470 L) / 0.160 L
M2 = 1.18 M
To know more about molarity, visit:
https://brainly.com/question/2817451
#SPJ1
write a balanced net ionic equation for the reaction of nibr2(aq) with (nh4)2s(aq).
Answers
The balanced net ionic equation for the reaction of NiBr2(aq) with (NH4)2S(aq) is:
Ni2+(aq) + S2-(aq) → NiS(s)
Note that the spectator ions NH4+ and Br- do not participate in the reaction and are not included in the net ionic equation.
Spectator ions are ions that do not participate in a chemical reaction, meaning they do not undergo any chemical change during the reaction. They are present in both the reactants and the products and do not affect the outcome of the reaction.
Spectator ions can be identified by looking at the balanced chemical equation of the reaction and canceling out ions that appear on both sides of the equation.
Visit here to learn more about spectator ions brainly.com/question/28913274
#SPJ11
The balanced net ionic equation for the reaction of NiBr2(aq) with (NH4)2S(aq) is:
Ni2+(aq) + S2-(aq) → NiS(s)
Note that the spectator ions NH4+ and Br- do not participate in the reaction and are not included in the net ionic equation.
Spectator ions are ions that do not participate in a chemical reaction, meaning they do not undergo any chemical change during the reaction. They are present in both the reactants and the products and do not affect the outcome of the reaction.
Spectator ions can be identified by looking at the balanced chemical equation of the reaction and canceling out ions that appear on both sides of the equation.
Visit here to learn more about spectator ions brainly.com/question/28913274
#SPJ11
An avalanche comes down the East side of the mountain. If the avalanche lasted for 97 seconds and travels 4.5 miles, what was the velocity of the avalanche?
Answers
The velocity of the avalanche is 0.04 miles / sec. If the avalanche lasted for 97 seconds and travels 4.5 miles,
What is Velocity ?Velocity is a vector expression of the displacement that an object or particle undergoes with respect to time .
The standard unit of velocity magnitude (also known as speed ) is the meter per second (m/s). Alternatively, the centimeter per second (cm/s) can be used to express velocity magnitude.
Given ;
Time = 97 secDistance = 4.5 milesFormula used ;
Velocity = Distance / Time
Therefore,
Velocity = 4.5 miles / 97 sec
= 0.04 miles / sec
Learn more about Velocity here ;
https://brainly.com/question/13639113
#SPJ1
The liter is a measurement of which of the following qualities volume,teampature,mass,density
Answers
Answer:
Volume
Explanation:
Volume is the quantity of three-dimensional space enclosed by a closed surface, for example, the space that a substance (solid, liquid, gas, or plasma) or 3D shape occupies or contains.[1] Volume is often quantified numerically using the SI derived unit, the cubic metre. The volume of a container is generally understood to be the capacity of the container; i.e., the amount of fluid (gas or liquid) that the container could hold, rather than the amount of space the container itself displaces. Three dimensional mathematical shapes are also assigned volumes. Volumes of some simple shapes, such as regular, straight-edged, and circular shapes can be easily calculated using arithmetic formulas. Volumes of complicated shapes can be calculated with integral calculus if a formula exists for the shape's boundary. One-dimensional figures (such as lines) and two-dimensional shapes (such as squares) are assigned zero volume in the three-dimensional space.
The volume of a solid (whether regularly or irregularly shaped) can be determined by fluid displacement. Displacement of liquid can also be used to determine the volume of a gas. The combined volume of two substances is usually greater than the volume of just one of the substances. However, sometimes one substance dissolves in the other and in such cases the combined volume is not additive.[2]
In differential geometry, volume is expressed by means of the volume form, and is an important global Riemannian invariant. In thermodynamics, volume is a fundamental parameter, and is a conjugate variable to pressure.
What masses of dimethylamine and dimethylammonium chloride do you need to prepare 8. 00 l of ph = 12. 00 buffer if the total concentration of the two components is 0. 500 m?
Answers
The masses of dimethylamine and dimethylammonium chloride do you need to prepare 8. 00 l of pH = 12. 00 buffer is 1448 gm.
What is a Buffer Solution ?
A buffer is an aqueous solution that can resist significant changes in pH levels upon the addition of small amount of acid or alkali.
dimethylamine (CH₃)₂NH and dimethylammonium chloride (CH₃)₂NH * HCl are mixed to prepare a buffer solution
The concentration of the components is given as 0.5M , Volume = 8L
no. of moles = CV = 0.5 * 8 =4 moles
4 moles of dimethylamine = mass/ Molecular weight
Molecular weight of dimethylamine = 45 gm
Therefore the mass of dimethylamine required = 45 * 4 = 180 gm
4 moles of dimethylammonium chloride = 362 gm
Therefore the mass of dimethylammonium chloride required = 362*4 = 1448 gm
Thus the masses of dimethylamine and dimethylammonium chloride do you need to prepare 8. 00 l of pH = 12. 00 buffer is 1448 gm.
To know more about Buffer Solution
https://brainly.com/question/3435382
#SPJ4
match each compound with the value of ksp expressed as function of the molar solubility fecl3 cabr2 fecl2
Answers
To match each compound with the value of Ksp expressed as a function of the molar solubility, we need to know the chemical equations for the dissolution of these compounds.
The molar solubility of a compound is the number of moles of the compound that dissolve in one liter of solvent.
The solubility product constant (Ksp) is an equilibrium constant that represents the extent of the dissolution of a sparingly soluble compound.
Here are the chemical equations for the dissolution of the compounds you provided:
1. FeCl3:
FeCl3(s) ⇌ Fe3+(aq) + 3Cl-(aq)
2. CaBr2:
CaBr2(s) ⇌ Ca2+(aq) + 2Br-(aq)
3. FeCl2:
FeCl2(s) ⇌ Fe2+(aq) + 2Cl-(aq)
Now, let's match each compound with the corresponding expression of Ksp in terms of molar solubility:
1. FeCl3:
The molar solubility of FeCl3 is [Fe3+] = x and [Cl-] = 3x. Since there are three chloride ions per formula unit of FeCl3, the expression for Ksp is:
Ksp = [Fe3+][Cl-]³ = x(3x)³ = 27x⁴
2. CaBr2:
The molar solubility of CaBr2 is [Ca2+] = x and [Br-] = 2x. Since there are two bromide ions per formula unit of CaBr2, the expression for Ksp is:
Ksp = [Ca2+][Br-]² = x(2x)² = 4x³
3. FeCl2:
The molar solubility of FeCl2 is [Fe2+] = x and [Cl-] = 2x. Since there are two chloride ions per formula unit of FeCl2, the expression for Ksp is:
Ksp = [Fe2+][Cl-]² = x(2x)² = 4x³
Therefore, the compounds matched with their corresponding expressions of Ksp in terms of molar solubility are:
1. FeCl3: Ksp = 27x⁴
2. CaBr2: Ksp = 4x³
3. FeCl2: Ksp = 4x³
To know more about molar solubility refer here
brainly.com/question/31043999#
#SPJ11
Water enters a system at T=130∘C and h=1700 kJ/kg. Work is then done on the system leaving exit conditions as T=155∘C and P=1MPa. Calculate the specific volume of the water at the inlet (m3/kg). Find the specific enthalpy at the exit (kJ/kg). Show where the inlet and exits states are on a T-v diagram.
Answers
The specific enthalpy of water vapor at 155°C and 1 MPa is 3326 kJ/kg.
The specific volume of the water at the inlet (m³/kg) is calculated as follows:
Given: Water enters the system at T=130°C and h=1700 kJ/kg.
Process: First, determine the specific volume of the water at the inlet. To do this, we need to consult the water table. We'll look for the table corresponding to the saturation temperature of 130°C (table A-4). It is observed that at 130°C, the specific volume of saturated liquid is 0.00121 m³/kg, which is the specific volume at the inlet. Next, determine the specific enthalpy of water at the exit. To do this, we need to consult the water table. We'll look for the table corresponding to the temperature of 155°C (Table A-4).At a temperature of 155°C, we find the saturation pressure to be 2.96 MPa, which is less than the given exit pressure of 1 MPa.
Therefore, the water is superheated, and we'll use Table A-5 instead of Table A-4.
Table A-5 shows the specific enthalpy of water vapor at different temperatures and pressures. Since the exit temperature is 155°C, we'll find the corresponding specific enthalpy of water vapor at 1 MPa using Table A-5. The specific enthalpy of water vapor at 155°C and 1 MPa is 3326 kJ/kg, which is the specific enthalpy at the exit states are represented on a T-v diagram. The specific volume at the inlet and the specific enthalpy at the exit are represented on the T-v diagram.
To know more about enthalpy of water visit:
https://brainly.com/question/20897184
#SPJ11
Enter the correct 4 digit code (no spaces) *
Answers
Answer:6969?
Explanation:
When heated to 350 degrees C at 0. 950 atm, the ammonium nitrate decomposes to produce nitrogen, water, and oxygen gases; 2NH4NO3(s) delta--->2N2(g)+4H2O(g)+O2(g): a) How many liters of water vapor are produced when 25. 8 g of NH4NO3 decomposes? b) How many grams of NH4NO3 are needed to produce 10. 0 L of oxygen?
Answers
25.8 g of NH₄NO₃ decomposed to produce 32.3 L of water vapor. 71.4 g of NH₄NO₃ are needed to produce 10.0 L of O₂.
a) To determine the number of liters of water vapor produced, we first need to calculate the moles of NH₄NO₃ that decompose:
The molar mass of NH₄NO₃ is:
M(NH₄NO₃) = 14.01 g/mol (N) + 4(1.01 g/mol) (H) + 3(16.00 g/mol) (O) = 80.05 g/mol
The moles of NH₄NO₃ can be calculated as:
moles NH₄NO₃ = mass/molar mass = 25.8 g / 80.05 g/mol = 0.322 moles NH₄NO₃
From the balanced equation, we see that 4 moles of H₂O are produced for every 2 moles of NH₄NO₃ that decompose, so we can calculate the moles of H₂O produced as:
moles H₂O = 4/2 x moles NH₄NO₃ = 4/2 x 0.322 = 0.644 moles H₂O
Finally, we can use the ideal gas law to calculate the volume of water vapor produced at 350 degrees C and 0.950 atm:
PV = nRT
V = nRT/P
V = (0.644 mol) (0.0821 L·atm/mol·K) (623 K) / (0.950 atm) = 32.3 L
Therefore, 25.8 g of NH₄NO₃ decomposed to produce 32.3 L of water vapor.
b) To determine the grams of NH₄NO₃ needed to produce 10.0 L of O2, we can use the same approach, starting with the ideal gas law:
The molar volume of a gas at standard temperature and pressure (STP) is 22.4 L/mol.
The moles of O2 needed to produce 10.0 L can be calculated as:
moles O2 = V/STP = 10.0 L / 22.4 L/mol = 0.446 moles O2
From the balanced equation, we see that 2 moles of NH₄NO₃ decompose to produce 1 mole of O2, so we can calculate the moles of NH₄NO₃ needed as:
moles NH₄NO₃= 2/1 x moles O2 = 2/1 x 0.446 = 0.892 moles NH4NO3
Finally, we can use the molar mass of NH4NO3 to calculate the grams needed:
mass NH₄NO₃ = moles NH₄NO₃ x molar mass = 0.892 mol x 80.05 g/mol = 71.4 g
Therefore, 71.4 g of NH₄NO₃ are needed to produce 10.0 L of O₂.
Learn more about ideal gas law ,
https://brainly.com/question/28257995
#SPJ4
Draw the Lewis dot diagrams for F2, N2, and C2. Compare your drawing to the ones below. Which of the below Lewis dot diagram or diagrams is/are wrong and why?

Answers
answer: i believe it is N2 is wrong because it has a double bond
Explanation:
i need help as well
¿Como pasar 254 meses a minutos?
Por favor ayudaaa!!
Le doy corona a la respuesta
Answers
Answer:
Ver las respuestas abajo.
Explanation:
Este problema se puede resolver conociendo la relacion entre horas y minutos, sabemos que:
1 hora [h] → 60 minutos [min]
De esta manera:
2 [min] = 2/60 = 0.033 [h]
15 [min] = 15/60 = 0.25 [h]
30 [min] = 30/60 = 0.5 [h]
10 [min] = 10/60 = 0.166 [h]
6 [min] = 6/60 = 0.1 [h]
20 [min] = 20/60 = 0.33 [h]
5 [min] = 5/60 = 0.0833 [h]
Select a description. *

Answers
Explanation:
this are compounds being that they are combined together
Which pair of elements has the most similar Lewis structures?
Group of answer choices
a)O and S
b)N and S
c)F and Ar
d)Cl and Ar
Answers
Answer: C.)
Explanation:
A clear colorless liquid in an open beaker was heated to boiling. The liquid began to boil at 110°C, and as vapors escaped, the temperature of boiling gradually increased to 115°C, at which point the heating was stopped. On the basis of this information, we can say that the material in the beaker was a
Answers
Omitted options and they are
a) pure compound.
b. pure element.
c. pure substance.
d. homogeneous solution.
e. heterogeneous solution
Answer:d. homogeneous solution.
Explanation:
Pure substances or elements or compounds have a definite and sharp melting or boiling point, Any substance that is not pure is impure and will have different temperature of melting or boiling points.
To this effect, the clear colorless liquid cannot be a Pure substance, element or compound.
We can therefore say that the clear colorless liquid would be a homogeneous solution because a homogeneous solution is a mixture of constituents which completely mixes together such that each constituents cannot be seen with naked eye, When heated to boiling, each constituent in the mixture will give different boiling points.
A heterogeneous Solution, too is a mixture but contains constituents that can be seen and not a clear colourless solution.
Therefore On the basis of this information, we can say that the material in the beaker was a Homogeneous solution
The substance must be a homogeneous solution since it is a colorless liquid.
The boiling point of a solution is the temperature at which the atmospheric pressure equals the pressure of the liquid. We must note that a pure substance has a sharp boiling point.
A homogeneous solution is a solution that constitutes only one phase. A solution usually boil over a range of temperature hence the substance must be a homogeneous solution since it is a colorless liquid.
Learn more about homogeneous solution: https://brainly.com/question/14454579
2.
Si la masse volumique d'une substance est 10g/ml et sa masse est 80g, quel est
son volume??
Answers
Answer:
C
Explanation:
#7) How many waves are in this picture?

Answers
Answer:
4
Explanation:
Answer:
B.
Explanation:
I don't know much about this subject, but it seems that a wave is when the line is above the line in the middle.
What causes streams to form?
O absorption
O precipitation
O glaciers
O lakes
Answers
Answer: essay the answer is B: precipitation
Explanation:
3.4 x 1023 atoms of Na in moles
Answers
The number of moles of sodium (Na) in 3.4 x 10^23 atoms is approximately 5.64 moles.
In the first paragraph, the main answer is that there are approximately 5.64 moles of sodium (Na) in 3.4 x 10^23 atoms.
Now, let's explain the calculation in the second paragraph. The mole is a unit of measurement used in chemistry to quantify the amount of a substance. One mole of any element contains Avogadro's number of atoms, which is approximately 6.022 x 10^23. In this case, we have 3.4 x 10^23 atoms of sodium (Na). To convert this into moles, we divide the number of atoms by Avogadro's number.
Mathematically, the calculation is as follows:
Moles of Na = (Number of atoms of Na) / (Avogadro's number)
Moles of Na = (3.4 x 10^23) / (6.022 x 10^23)
Moles of Na ≈ 5.64 moles
Therefore, there are approximately 5.64 moles of sodium (Na) in 3.4 x 10^23 atoms.
for such more questions on moles
https://brainly.com/question/29367909
#SPJ8
The regiochemistry of hydroboration/oxidation of alkenes is: (a) Markovnikov (b) non-Markovnikov (c) subject to solvent effects (d) unrelated to alkene structure (e) it is a not a regiospecific reaction.
Answers
The regiochemistry of hydroboration/oxidation of alkenes is (a) Markovnikov.
The hydroboration/oxidation reaction follows Markovnikov's rule, which states that the electrophile (in this case, the boron atom) adds to the carbon atom of the double bond that has the greater number of hydrogen atoms attached to it. The regioselectivity of the reaction is determined by the relative stability of the carbocation intermediates formed during the reaction.
In hydroboration, the boron atom adds to the less substituted carbon atom of the double bond, leading to the formation of a boron-alkyl bond and a boron-hydrogen bond. Subsequently, in the oxidation step, the boron-alkyl bond is replaced with an alcohol group (-OH) while maintaining the regiochemistry established during hydroboration.
Therefore, the regiochemistry of hydroboration/oxidation of alkenes is Markovnikov, where the electrophilic addition occurs preferentially at the carbon atom of the double bond that has the greater number of hydrogen atoms attached to it.
To learn more about alkenes here:
https://brainly.com/question/30217914
#SPJ11
calculate the heat of reaction delta h for the following reaction: ccl4(g) h2o(g) -> chcl3(g) hcl(g)
Answers
The heat of reaction (ΔH) for the given reaction is 180.4 kJ/mol. To calculate the heat of reaction (ΔH) for the given reaction:
CCl₄(g) + H₂O(g) -> CHCl₃(g) + HCl(g)
You would need the standard enthalpies of formation for each compound involved in the reaction. The standard enthalpy of formation (ΔHf) is the enthalpy change when one mole of a compound is formed from its elements in their standard states.
Here are the standard enthalpies of formation for the compounds involved:
ΔHf[CCl₄(g)] = -135.5 kJ/mol
ΔHf[H₂O(g)] = -241.8 kJ/mol
ΔHf[CHCl₃(g)] = -104.7 kJ/mol
ΔHf[HCl(g)] = -92.3 kJ/mol
To calculate ΔH for the reaction, you need to sum up the enthalpies of formation of the products and subtract the sum of the enthalpies of formation of the reactants:
ΔH = ΣΔHf(products) - ΣΔHf(reactants)
ΔH = [ΔHf[CHCl₃(g)] + ΔHf[HCl(g)]] - [ΔHf[CCl₄(g)] + ΔHf[H₂O(g)]]
ΔH = [(-104.7 kJ/mol) + (-92.3 kJ/mol)] - [(-135.5 kJ/mol) + (-241.8 kJ/mol)]
ΔH = -196.9 kJ/mol - (-377.3 kJ/mol)
ΔH = 180.4 kJ/mol
Therefore, the heat of reaction (ΔH) for the given reaction is 180.4 kJ/mol.
To know more about enthalpy:
https://brainly.com/question/29145818
#SPJ4
HURRY ANSWER NOW AND I GIVE BRAINLY!!! HURYYY
1. A student left a sealed jar of water outside her home. Water can be a solid, liquid, or gas. When she put it outside, the water was a solid. Twelve hours later, the water had changed phase and was a liquid. What happened to the water molecules?
Answers
Answer:
A. "before the student left, the molecules we're moving in place. After, the molecules we're moving around each other.
Explanation:
Hope this helps. Please mark as brainliest if it did. :)
how to scientists know where to put electrons in the atom
a. probability
b. ratio and proportions
c. algebra
d. geometry
Answers
Answer:
a).
Explanation:
precise electron locations cannot be calculated, only predicted based on previous calculations. they cannot know whether or not an electron will be or will not be somewhere specific
have a nice day!
if 12.23 g of bromomethane are produced when 5.00 g of methanol is reacted with excess hbr, what is the percentage yield?
Answers
The percentage yield of bromoethane is 82.45%.
The percentage yield of a reaction can be calculated using the following formula:
Percentage Yield = (Actual Yield / Theoretical Yield) x 100
For this reaction, the theoretical yield of bromomethane is calculated by multiplying the moles of methanol by the moles of bromomethane and its molar mass.
Theoretical Yield = 5.00 g/32.04 g/mol x 1mol x 95g = 14.834 g bromomethane
where 95g is the molar mass of bromomethane.
The actual yield is given as 12.23 g, so the percentage yield is calculated as:
Percentage Yield = (12.23 g/14.834 g) x 100 = 82.45%
Therefore, the percentage yield of bromoethane in the reaction is 82.45%.
To know more about bromoethane, refer here:
https://brainly.com/question/29819429#
#SPJ11
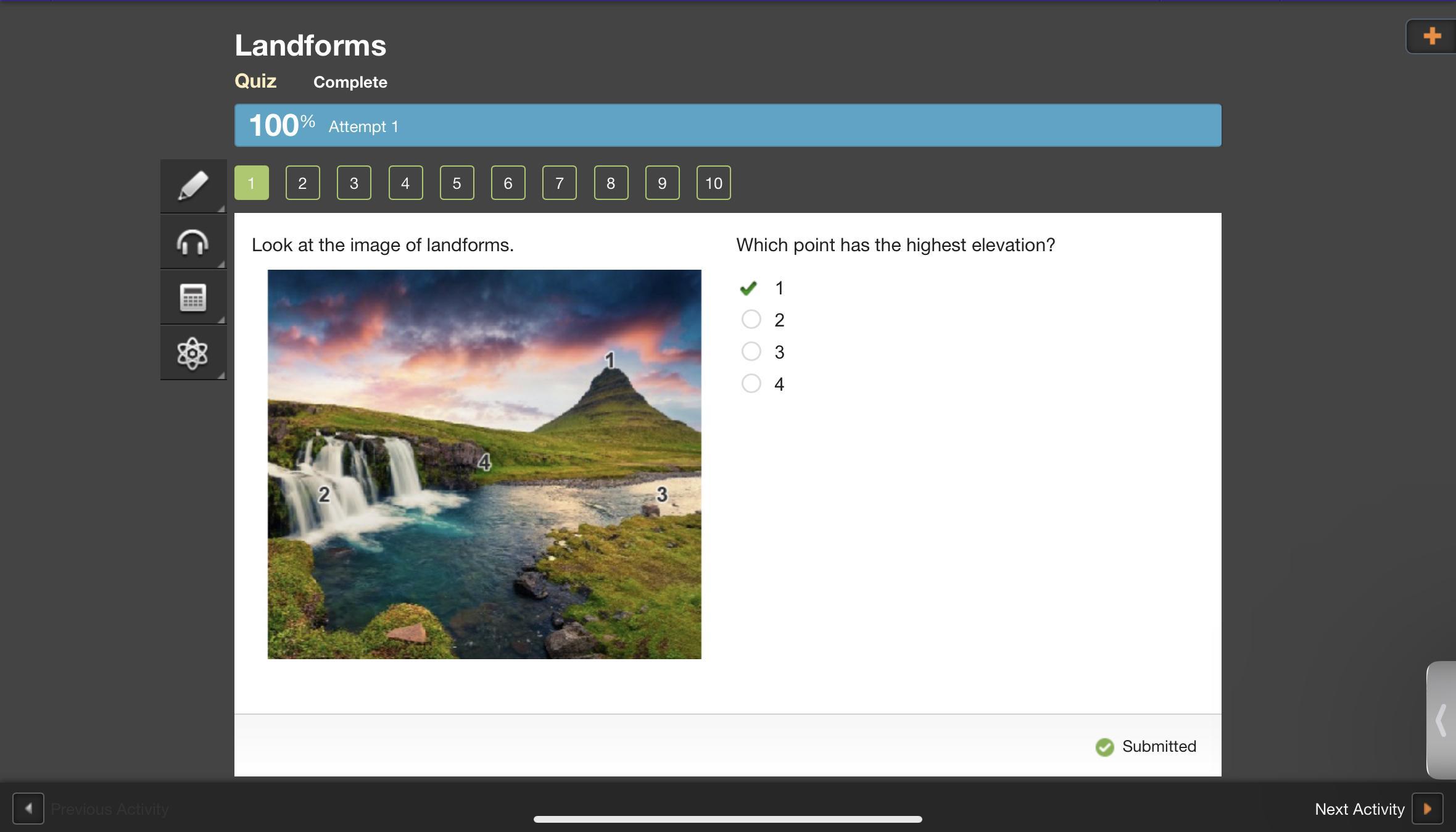
look at the image of landforms which point has the highest elevation
Answers
And I’ll help
Answer: a. 1
Explanation:
Physical crowding and loud noise are considered sources of ___________ and are linked to the incidence of increased stress hormones.
Answers
Physical crowding and loud noise are considered sources of environmental stressors and are linked to the incidence of increased stress hormones.
High concentration of cytoskeletal filaments, organelles, and proteins along with the space constraints due to the axon’s narrow geometry lead inevitably to intracellular physical crowding along the axon of a neuron. . Molecular motors that mediate active transport share movement mechanisms that allow them to bypass physical crowding present on microtubule tracks. Everyday loud noise typically do not damage your hearing. However, many people participate in activities that produce harmful sound levels, which repeated over time will cause hearing loss.
learn more about:- Physical crowding & loud noise here
https://brainly.com/question/31149746
#SPJ11
The manhattan project was responsible for the creation of _____. the atomic bomb the hydrogen bomb the first satellite the aswan dam
Answers
Which bomb was created as a part of the manhattan project?
The manhattan project was responsible for the creation of the atomic bomb.
What was the manhattan project?
The first nuclear weapons were created as a result of the Manhattan Project's research and development efforts during World War II. With the help of the United Kingdom and Canada, it was spearheaded by the United States. Major General Leslie Groves of the U.S. Army Corps of Engineers oversaw the project from 1942 to 1946. Robert Oppenheimer, a nuclear physicist, served as the lab's director and was responsible for the actual bomb's design, the atomic bomb.
As the Army's first headquarters were located in Manhattan, the project's Army component was given the moniker Manhattan District, gradually replacing the official codename for the entire project, Development of Substitute Materials. The project eventually absorbed Tube Alloys, its previous British counterpart. The Manhattan Project started out small in 1939, but it eventually employed over 130,000 people and cost close to US$2 billion (about $23 billion in 2020). Less than 10% of the cost went toward the design and production of the weapons, with more than 90% going toward the construction of factories and the production of fissile material. More than thirty locations in the United States, the United Kingdom, and Canada were used for research and production.
Learn more about the manhattan project here,
https://brainly.com/question/1129537
#SPJ4