An R-32 vapor-compression system provides 4 tons of cooling, has 10∘C liquid subcooling, 8∘C superheated vapor, an evaporator temperature of 6∘C, and a condensing temperature of 58∘C. The compressor isentropic efficiency is 70%. Neglect all component pressure drops, and determine a. The COP b. The compressor discharge temperature
Answers
The Coefficient of Performance (COP) of the R-32 vapor-compression system is 4.18 and the compressor discharge temperature is 1491.9 °C.
Given, the cooling capacity = 4 tons or 48,000 Btu/hr
Liquid subcooling = 10∘C
Superheated vapor = 8∘C
Evaporator temperature = 6∘C
Condensing temperature = 58∘C
Isentropic efficiency of compressor = 70%
Neglect all component pressure drops.
Solution
a) Coefficient of Performance (COP)
COP is defined as the ratio of cooling capacity to the compressor work input.
The cooling effect produced by a refrigeration system is expressed in tons of refrigeration.
1 ton of refrigeration = 12,000 Btu/hr
Cooling capacity = 4 tons or 48,000 Btu/hr
COP = (cooling effect produced) / (work input of compressor)
COP = (48,000) / Wc ...........(1)
To calculate Wc, we can use the first law of thermodynamics.
In a steady-flow system, the net work produced is equal to the change in the enthalpy (H) of the system.
So, Wc = H2 - H1
Wc = m × (h2 - h1)
Wc = m × [(H2 - H1) / m]
Wc = (H2 - H1) / η
Isentropic efficiency η = 70% or 0.7
ΔH = H2 - H1
Enthalpy values can be obtained from the refrigerant property tables for R-32 at respective temperatures.
Wc = ΔH / η
Wc = (H2 - H1) / 0.7
Substituting Wc in equation (1)
COP = (48,000) / [(H2 - H1) / 0.7]
At evaporator condition, refrigerant exists as superheated vapor at 8∘C.
So, we can obtain the enthalpy value from the refrigerant property tables for R-32 at 8∘C.
H1 = 1.1343 kJ/
At condenser condition, refrigerant exists as saturated vapor at 58∘C.
So, we can obtain the enthalpy value from the refrigerant property tables for R-32 at 58∘C.
H2 = 242.4 kJ/kg
Substituting the values
H1 = 1.1343 kJ/kg,
H2 = 242.4 kJ/kg
COP = (48,000) / [(242.4 - 1.1343) / 0.7]
COP = 4.18
b) Compressor discharge temperature
Compressor work input can be determined as follows,
Wc = ΔH / η
ΔH = H2 - H1
ΔH = 242.4 - 1.1343
ΔH = 241.27 kJ/kg
Wc = 48,000 / 4.18
Wc = 11,484.6 Btu/hr
We know that compressor work input is equal to the change in enthalpy of the refrigerant during compression.
So, the discharge temperature of the compressor can be calculated by using refrigerant property tables for R-32 at respective conditions.
The refrigerant leaves the compressor as superheated vapor.
Compressor discharge temperature = Evaporator temperature + rise in temperature due to compression
T2 = T1 + ΔT......(2)
ΔT = (H2 - H1) / m × Cp......(3)
Cp = Specific heat capacity of R-32 at constant pressure
At evaporator condition, refrigerant exists as superheated vapor at 8∘C.
So, we can obtain the specific heat capacity value from the refrigerant property tables for R-32 at 8∘C.
Cp = 1.2551 kJ/kg·
K Substituting the values in equation (3)
ΔT = (241.27 / 0.7) × 1.2551 / 0.1365
ΔT = 1,758.9 K
Substituting the values in equation (2)
T2 = 6 + 1,758.9 = 1764.9 K
Compressor discharge temperature = 1764.9 - 273
Compressor discharge temperature = 1491.9 °C
to know more about compressor visit:
https://brainly.com/question/22170796
#SPJ11
Related Questions
HELP PLEASEE 100 POINTS
The Quiver tree grows in Southern Africa. Which of the following plant adaptations is likely to prevent these trees from dying out due to rising desert temperatures?
O Releasing a black powder onto their trunk to absorb more heat from sunlight
O Shifting their growing range towards the equator
Ability to store water in leafy structures to prevent excess evaporation
O Limiting seed dispersal to nearby locations
PLEASE HELP 100 POINTS !!
Answers
Answer:
Shifting their growing range towards the equator
Explanation:
Maybe will be like that
Answer:
Ability to store water in leafy structures to prevent excess evaporation.
Explanation:
The reason why plants die in hot temperature is the excess evaporation of water, so to prevent the excess evaporation of water, the plants get adaptive to store water in their leaves.
Hope it helps.
Saliva contains ____(bile,gastrin,or amylase) which helps digest ____.(protein,starch,or fat)
PLS HELP FOR A TEST!!
Answers
Answer:
Saliva contains amylase which helps digest starch .
Explanation:
you should've googled it instead of waiting 10,000 years for someone to answer. but sometimes googling doesn't always give you the answer you want
In lab students are going to burn strips of magnesium. If oxygen is needed to burn the magnesium in a synthesis reaction, what would this chemical equation be
Answers
The chemical equation for the synthesis reaction of burning magnesium in the presence of oxygen can be summarized as: 2 Mg + O2 → 2 MgO
In this reaction, magnesium (Mg) reacts with oxygen (O2) to form magnesium oxide (MgO). The balanced equation shows that two moles of magnesium react with one mole of oxygen to produce two moles of magnesium oxide.
When magnesium is burned, it undergoes a redox reaction with oxygen. Magnesium atoms lose two electrons to form Mg2+ ions, while oxygen molecules gain four electrons to form O2- ions.
The resulting ions combine to form the ionic compound magnesium oxide (MgO), which is a white solid. The balanced equation reflects the stoichiometry of the reaction, indicating the correct ratio of reactants and products.
To learn more about chemical equation click here: brainly.com/question/28792948
#SPJ11
How much energy is released when 67.04g of phosphorous is reacted with 10.20g of chlorine? ___ P + ___ Cl2 ___ PCl3 ΔH = -574 kJ
Answers
Answer:
26.78 kJ
Explanation:
To solve the problem, we have to first write the stoichiometric coefficients in the chemical equation:
2P + 3Cl₂ → 2PCl₃
With these coefficients, we have the same number of atoms of each chemical element on both sides: 2 atoms of P, 6 atoms of Cl.
According to the equation, 2 moles of phosphorous (P) react with 3 moles of chlorine (Cl₂), and 574 kJ of energy are released. We have to figure out which is the limiting reactant. For this, we convert the mass into moles by using the molar mass(MM):
MM(P) = 30.9 g/mol
67.04 g P/(30.9 g/mol) = 2.17 mol P
MM(Cl₂) = 35.4 g/mol x 2 = 70.8 g/mol
10.20 g Cl₂/(70.8 g/mol) = 0.14 mol Cl₂
Now, we multiply the actual moles of P (the amount we have for the reaction) by the stoichiometric ratio given by the chemical equation (3 mol Cl₂/2 mol P):
2.17 mol P x (3 mol Cl₂/2 mol P) = 3.25 mol Cl₂
To completely react 67.04 g P, we need 3.25 mol of Cl₂, and we have only 0.14 moles of Cl₂, so the limiting reactant is Cl₂.
Now, we use the limiting reactant to calculate the energy released from the reaction. The energy released per mole of Cl₂ is:
ΔH/(3 mol Cl₂) = -574 kJ/3 mol Cl₂= 191.3 kJ/mol Cl₂
Finally, we multiply the energy released per mole of Cl₂ by the number of moles of Cl₂ we have:
0.14 mol Cl₂ x 191.3 kJ/mol Cl₂ = 26.78 kJ
write the equilibrium constant (ksp) expression for the following equation: ca(oh)2 (s) ↔ ca2 (aq) 2oh- (aq)
Answers
The equilibrium constant (Ksp) expression for the given equation is as follows: Ksp = [Ca²⁺][OH⁻]²
Where [Ca²⁺] is the concentration of calcium ions in the solution and [OH⁻] is the concentration of hydroxide ions in the solution.
The Ksp value represents the solubility product of calcium hydroxide and is a measure of the extent to which the solid compound dissolves in water to form its constituent ions. A high Ksp value indicates that the compound is highly soluble in water, whereas a low Ksp value indicates that the compound is relatively insoluble.
In summary, the Ksp expression for the given equation is a measure of the solubility of calcium hydroxide in water and is given by the concentration of its constituent ions in the solution.
To know more about equilibrium constant, refer
https://brainly.com/question/3159758
#SPJ11
is RbCl soluble or insoluble in water
Answers
Answer:
Rubidium Chloride is an excellent water soluble crystalline Rubidium source for uses compatible with chlorides.
Compound Formula: ClRb
Melting Point: 715 °C
Boiling Point: 1,390 °C
Explanation:
Use the data table to answer the question that follows.
Production Possibilities for One Year
Cheese
(millions of tons)
United States
Italy
Tomatoes
(millions of tons)
2
1
EEEEE
Which statement is supported by the data table?
O The United States should produce neither cheese nor tomatoes but rather import them.
The United States should produce all its own cheese and tomatoes for consumption.
The United States should specialize in tomatoes and trade them for cheese from Italy.
O The United States should specialize in cheese and trade it for tomatoes from Italy.
Answers
On this scenario, the US may consume more than its PPC by specializing in cheese and swapping it for Italian tomatoes.
What makes this possible?Italy’s cheese output is lower than that of the United States.Italy cannot consume cheese other than PPC unless these products are supplied by another nation. Through an exchange deal with Italy, the United States can enjoy tomatoes in addition to PPC. Because cheese production in the United States is substantially higher than in Italy, we may assume that the United States can offer this commodity to other nations without jeopardizing its consumption. However, tomato output in the United States is the same as in Italy, but because the United States is a larger country than Italy, tomato consumption in the country may be affected.
As a result, if tomatoes are obtained from another nation, tomato consumption in the United States may surpass the PPC of tomatoes. This may be accomplished through barter, with the United States supplying cheese to Italy and Italy supplying tomatoes to the United States.
To learn more about PPC to refer:
https://brainly.com/question/16969732
#SPJ1
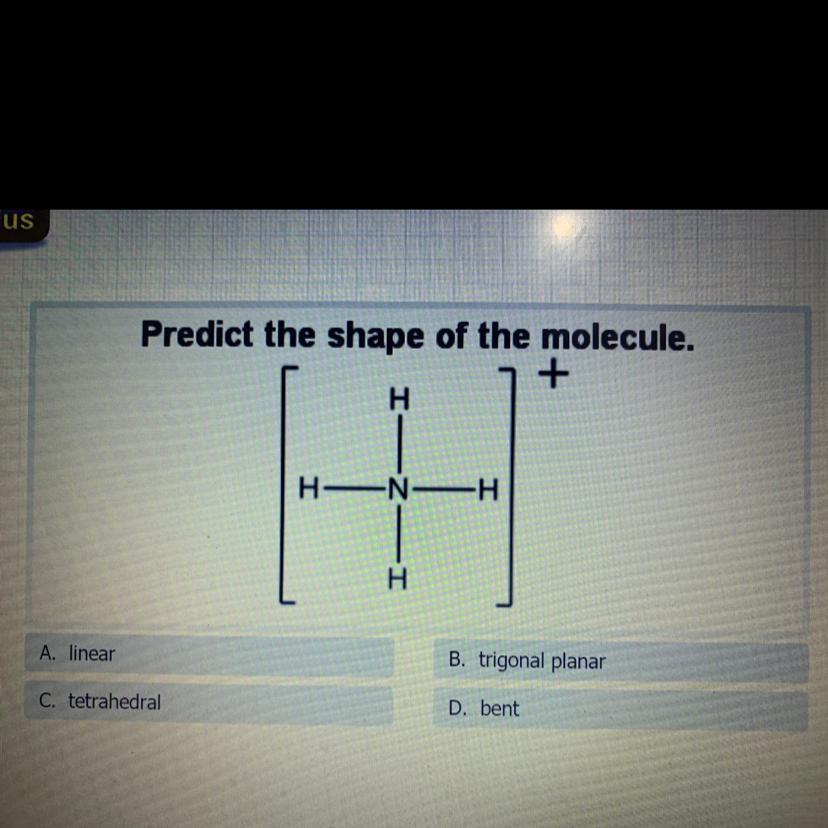
Predict the shape of the molecule.
B. trigonal planar
A. linear
D. bent
C. tetrahedra
Answers
Which are characteristics of a prokaryotic cell? Select three options. contains DNA lacks DNA contains ribosomes lacks ribosomes contains a nucleus lacks a nucleus
Answers
Answer:
Prokaryotic cells contain DNA, contain ribosomes, and lack a nucleus.
Answer:
Contains DNA
Contains Ribosomes
lacks a nucleus
Explanation: I took the quiz on edge
What are the products of combustion of hydrocarbons and water?.
Answers
Water and carbon dioxide are the byproducts of hydrocarbon burning.
What are some examples of hydrocarbons?A mixture of hydrocarbon is called a hydrocarbon. The hydrocarbons methane (CH4), ethane (C2H6), and ethyne are examples of (C2H2). These compounds are all hydrocarbons since their corresponding molecular formulas show that they are all solely composed of the elements hydrogen and carbon.
The biggest hydrocarbon is?The biggest and most intricate carbon molecules are found in coal. Various hydrocarbons produce different amounts of water and carbon dioxide because their hydrogen to carbon ratios vary. The ratio of charcoal to hydrogen is typically higher in longer, more complicated molecules.
To know more about Hydrocarbons visit:
https://brainly.com/question/12433190
#SPJ4
what do scientists think will happen if the earth's atmospheric carbon dioxide levels are doubled?
Answers
Doubling Earth's atmospheric CO₂ levels would lead to significant and potentially devastating changes to our planet's climate, ecosystems, and human well-being.
If Earth's atmospheric carbon dioxide (CO₂) levels were to double, scientists predict several significant consequences for our planet. Firstly, a major increase in global temperatures would occur due to the greenhouse effect. CO2, being a greenhouse gas, traps heat within Earth's atmosphere, leading to a rise in average temperatures, known as global warming.
This increase in temperature would result in the melting of polar ice caps and glaciers, causing a rise in sea levels. This, in turn, would lead to increased coastal flooding and the potential loss of habitats and infrastructure in low-lying areas. Additionally, weather patterns could become more extreme and unpredictable, with increased occurrences of droughts, storms, and floods, causing negative impacts on agriculture and ecosystems.
Moreover, higher CO₂ levels would lead to ocean acidification, a process wherein CO₂ dissolves in seawater, creating carbonic acid. This change in ocean chemistry would have severe consequences for marine life, particularly for organisms with calcium carbonate shells, such as corals, mollusks, and some plankton species, affecting the entire marine food chain.
Lastly, increased CO₂ levels would impact human health, with higher temperatures exacerbating air pollution, causing respiratory issues, and worsening existing health conditions.
To know more about ocean acidification, refer to the link below:
https://brainly.com/question/7604502#
#SPJ11
The half-life of tritium (h) is 12 years. how long does it take for 16.0 ng of tritium to decay to the point where 2.0 ng remains?
Answers
It takes 24 years for 16.0 ng of tritium to decay to the point where 2.0 ng remains.
Tritium has a half-life of 12 years, which means that in each 12-year period, half of the tritium atoms will decay. To calculate the time it takes for a specific amount of tritium to decay, we can use the concept of half-life.
Step 1:
In the first 12 years, half of the tritium will decay, leaving 8.0 ng remaining (16.0 ng / 2).
Step 2:
In the next 12 years (the second half-life), half of the remaining tritium will decay again, leaving 4.0 ng (8.0 ng / 2).
Step 3:
Continuing this pattern, after another 12 years (the third half-life), we have 2.0 ng remaining (4.0 ng / 2).
Therefore, it takes 24 years for 16.0 ng of tritium to decay to the point where 2.0 ng remains.
Learn more about decay
https://brainly.com/question/32086007
#SPJ11
How do you separate alcohol and water ?
Answers
Answer:
liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points. When the mixture is heated, one liquid evaporates before the other.
Explanation:
hope this helps :3
Determine how many mg are in 3.6 x 10^24 molecules of carbon tetrachloride. Write your
answer in scientific notation.
Answers
Answer:
919,000 mg CCl4
Explanation:
1 mole of any compound contains 6.023x10^23 molecules of that compound.
Use this relationship as a conversion factor: (6.022x10^23 molecules)/1 mole
(3.6 x 10^24 molecules of carbon tetrachloride)/((6.022x10^23 molecules)/1 mole) = 5.98 moles of carbon tetrachloride, \(CCl_{4}\)
The molar mass of CCl4 is 153.8 grams/mole
(5.98 moles)(153.8 grams/mole) = 919 grams, or 919,000 mg CCl4
Ea for the following uncatalyzed reaction is . Ea for the same reaction when catalyzed is .
(a) What is the ratio of the rate constant for the catalyzed reaction to that for the uncatalyzed reaction at ? Assume that the frequency factor is the same for each reaction
Answers
The ratio of the rate constant for the catalyzed reaction to that for the uncatalyzed reaction can be determined based on the activation energies of the reactions.
What is the ratio of the rate constants?The ratio of the rate constant for the catalyzed reaction (k_cat) to that for the uncatalyzed reaction (k_uncat) can be calculated using the Arrhenius equation:
\(\[ \frac{k_{cat}}{k_{uncat}} = \frac{e^{-\frac{E_{a,cat}}{RT}}}{e^{-\frac{E_{a,uncat}}{RT}}} \]\)
Where Ea,cat is the activation energy for the catalyzed reaction, Ea,uncat is the activation energy for the uncatalyzed reaction, R is the gas constant, and T is the temperature in Kelvin.
Assuming that the frequency factor (A) is the same for both reactions, it cancels out when calculating the ratio. Therefore, the ratio of the rate constants is solely dependent on the activation energies.
Learn more about rate constant
brainly.com/question/20305922
#SPJ11
How would you find the density of a can of soda pop?
A. Find the mass of the can of soda pop and then multiply by the number of cubic centimeters in the can
B. Find the mass of the can of soda pop and then divide by the number of cubic centimeters in the can
C. Convert a gallon into cubic centimeters and then divide by the mass of the can of soda pop
D. Convert a gallon into cubic centimeters and then subtract the mass of the can of soda pop
Answers
Answer:
it's A.
Explanation:
have uh good day ma :)))))))
How to Represent nitrogen and magnesium with the Bohr-Rutherford model?
Answers
Nitrogen: 2 electron shells and 5 valence electrons
Magnesium: 3 electron shells 2 valence electrons
circulating energy substrates include glucose, fatty acids and amino acids, as well as ketone bodies and ______.
Answers
Answer: lactate
Explanation:
The missing circulating energy substrate is lactate. Lactate is a product of anaerobic metabolism and can serve as an energy source in certain conditions, such as during intense exercise or in tissues with high glycolytic activity. It can be converted back into glucose through a process known as the Cori cycle and utilized as an energy substrate by various tissues in the body.
In a chemical reaction which cannot occur:
Select one:
a. new elements are formed
b. heat is released
c. light is produced
d. a temperature change
Answers
Explanation:
Light can't get produced whilst a chemical reaction.
How do weathering and deposition differ? Weathering breaks down rocks; deposition leaves them in new places. Weathering has to do with air; deposition has to do with plants. Weathering occurs only in summer; deposition occurs year-round. Weathering can be chemical or physical; deposition is only chemical
Answers
Answer:
A. Weathering breaks down rocks; Deposition leaves them in new places.
Explanation:
Weathering is basically the complete process of rocks breaking apart. In contrast, deposition is when the rocks are moved and carried away from their original place and put in new locations.
Answer:
a
Explanation:
how many carbons are in cholesterol
Answers
Answer:
Cholesterol is a 27 carbon
when a scientist is beginning the process what is the key term she or he must ask in order to begin?
A: when or where
B:why or how
C: who or how many?
D: which or what
plz help thank you
Answers
Answer:
B- why or how because any scientist deals with matter it's relationships ,properties and its composition which can be inferred from the questions why and how
Why is it beneficial for chemists to understand as many periodic trends as they can?
Answers
Answer:
It can help identify elements quicker.
Explanation:
3.00g of Cacl^2 is dissolved in 100.0mL of Water. What is the mass percentage of Cl- in the solution?Use the filling values to calculate: MM of Ca = 40.078 g/mol MM of Cl = 35.45 g/mol Density of Water: 1.00g/mL
Answers
The mass percentage of Cl⁻ in the solution produced when 3.00g of CaCl₂ is dissolved in 100.0mL of water is 1.86 %.
What is the total mass of the given solution?The total mass of the solution which is produced when 3.00g of CaCl₂ is dissolved in 100.0mL of water is determined as follows:
mass of CaCl₂ = 3.0 g
mass of water = density * volume
mass of water = 1.00 g/mL * 100.0 mL
mass of water = 100.0 g
mass of solution = (100.0 + 3.0) g
total mass of solution = 103.0 g
molar mass of CaCl₂ = (40 + 35.5 * 2) g/mol
molar mass of CaCl₂ = 111 g/mol
mass percentage of Cl⁻ in CaCl₂ = 71/111
mass of Cl⁻ present in 3.0 g of CaCl₂ = 71/111 * 3 = 1.92 g
mass percentage of Cl⁻ in the solution = 1.92/103 * 100%
mass percentage of Cl⁻ in the solution = 1.86 %
Learn more about mass percent at: https://brainly.com/question/23856068
#SPJ1
Describe the energy in natural gas and the way in which it’s converted to electrical energy.
Answers
Which organism relies upon organic compounds for its carbon and energy needs?
Answers
Answer: Your welcome!
Explanation:
All living organisms rely on organic compounds for their carbon and energy needs. Organic compounds are molecules that contain carbon atoms and usually hydrogen, oxygen, and other elements. These organic compounds provide the necessary elements for cellular metabolism and are used to form cell structures and to store energy. Examples of organic compounds used by organisms include carbohydrates (glucose, glycogen, etc.), lipids (fats, oils, etc.), proteins, and nucleic acids (DNA, RNA, etc.).
Answer:
Chemoheterotrophs
Explanation:
which of the following best describes a result of the polar nature of water molecules? water molecules repel each other. the volume of water decreases by nearly half when it is frozen. water molecules repel most other substances. ionic compounds dissolve easily in water.
Answers
In water, ionic substances dissolve quickly. This sentence outlines how water molecules are polar nature. A polar covalent molecule is water.
Due to the unequal sharing of electrons between the atoms and the molecule's (side) asymmetrical structure, a water molecule has two poles: a positive charge on the hydrogen pole (side) and a negative charge on the oxygen pole. The water molecule has a curved form, which means that the majority of the negative charge from the oxygen is on one side of the molecule and on the other atoms positive charge from hydrogen is present. As a result, the water molecule can be described as polar.
To learn more about polar nature click here https://brainly.com/question/28158076
#SPJ4
the volume of gas at a stp is ..................l
Answers
Answer:
22.4 L
Explanation:
At STP, one mole of gas occupies 22.4 L of volume (molar volume). Note the International Union of Pure and Applied Chemistry (IUPAC) applies a more stringent standard of STP as a temperature of 273.15 K (0 °C, 32 °F) and an absolute pressure of exactly 100,000 Pa (1 bar, 14.5 psi, 0.98692 atm).
Answer:5.3 a
letr
Explanation:
The active ingredient in a Tum® antacid tablet i calcium carbonate (CaCO 3, FM = 100. 09), it neutralize exce hydrochloric acid (HCl, FM = 36. 46) in the tomach via the reaction CaCO 3 () 2HCl(aq) → CaCl 2 (aq) H 2 O(l) CO 2 (g). A certain doe of Tum containing 750 mg of CaCO 3 i added to 25 mL of 0. 100 M HCl. What i the volume of CO 2 generated under condition of STP?
Answers
The volume of CO₂ generated under condition of STP in the reaction :
CaCO₃ + 2HCl -----> CaCl₂ + H₂O + CO₂ is 12.5 mL.
The reaction is given as :
CaCO₃ + 2HCl -----> CaCl₂ + H₂O + CO₂
mass of CaCO₃ = 750 mg = 0.75 g
molarity of HCl = 0.100 M
volume of HCl = 25 mL = 0.025 L
moles of CaCO₃ = 0.75 / 100
= 0.0075 mol
moles of HCl = 0.100 × 0.025
= 0.0025 mol
here HCl is limiting reagent , formation of CO₂ depends on HCl
2 moles of HCl = 1 mole of CO₂
0.0025 mol of HCl = 0.0025 / 2
= 0.00125 mol
volume of CO₂ = moles / molarity
= 0.00125 / 0.100
= 0.0125 L = 12.5 mL
To learn more about moles here
https://brainly.com/question/29724957
#SPJ4
Which pod would have a greater change in velocity if you exerted the same strength force, a less massive pod or a more massive pod
