An inflated balloon is left outside overnight. initially it has a volume of 1.84 L when the temperature is 293.4 K and the pressure. what temperature will the balloon have a volume of 1540mL. If the pressure falls to 14.41 psi?
Answers
Answer:
ffff
Explanation:
Related Questions
Density = Mass / Volume Density = 35.062g / 23.50 mL =
Answers
Answer:
Density = 1.492g/mL
Explanation:
hope that will help you
Substances with a high polarity have a(low/high) surface tension ?
Answers
Answer:
I think they have high, because the nonpolar molecules usually have a much lower surface tension than polar ones.
calculate the permitted values of j for (a) a p electron and (b) an h electron.
Answers
The permitted values of j for a p electron are 1/2 and 3/2 and the permitted values of j for an h electron are 9/2 and 11/2.
(a) For a p electron:
The azimuthal quantum number (l) for a p electron is 1. To calculate the permitted values of j, we use the formula:
j = l ± 1/2
So for a p electron, the permitted values of j will be:
j = 1 + 1/2 = 3/2
j = 1 - 1/2 = 1/2
Therefore, the permitted values of j for a p electron are 1/2 and 3/2.
(b) For an h electron:
The azimuthal quantum number (l) for an h electron is 5. To calculate the permitted values of j, we use the same formula:
j = l ± 1/2
So for an h electron, the permitted values of j will be:
j = 5 + 1/2 = 11/2
j = 5 - 1/2 = 9/2
Therefore, the permitted values of j for an h electron are 9/2 and 11/2.
Learn more about permitted values at
brainly.com/question/28392032
#SPJ11
2) the quantity of antimony in a sample can be determined by an oxidation-reduction titration with an oxidizing agent. a 9.62 g sample of stibnite, an ore of antimony, is dissolved in hot, concentrated hcl(aq) and passed over a reducing agent so that all the antimony is in the form sb3 . the sb3 (aq) is completely oxidized by 43.70 ml of a 0.1250 m solution of kbro3. calculate the amount of antimony in the sample and its percentage in the ore.
Answers
Based on the results of the titration, there are 1.995 g of antimony in the stibnite sample, and its percentage is 20.7%.
In order to solve this, we need to write the balanced ionic reaction equation used in the titration:
3Sb³⁺(aq) + BrO₃⁻(aq) + 6H⁺(aq) → 3Sb⁵⁺(aq) + Br⁻(aq) + 3H₂O(l)
From the equation, we can tell that one mole of bromate (BrO₃⁻) reacts with 3 moles of Sb³⁺. To calculate the amount of Sb³⁺ from the sample, we must first calculate the number of moles (n) using the molarity (c = 0.1250 M) and the volume (43.70 mL = 0.0437 L) of the bromate solution.
c = n/V ⇒ n = c * V
n = 0.1250 M * 0.0437 L = 0.0054625 mol
This means that the amount of Sb³⁺ in the sample is 3 * 0.0054625 mol = 0.0163875 mol
To calculate the mass (m) of antimony in the sample, we will use the molar mass (M) of antimony (121.76 g/mol).
n = m/M ⇒ m = n*M
m = 0.0163875 mol * 121.76 g/mol = 1.995 g of antimony in the ore
Finally, we can calculate the percentage of antimony in stibnite:
%weight = 100% * m(antimony) / m(sample)
%weight = 100% * 1.995 g / 9.62 g = 20.7%
You can learn more about titrations here:
brainly.com/question/2728613
#SPJ4
how many kilojoules is 1,500,000 calories
Answers
Answer:
1 cal = 0.004187 kJ
1,500,000 cal = 6280.5 kJ
FOUR ways to obtain a brighter lamp?
Answers
Increasing LED brightness with a lampshade or reflection and choosing LED lights with high luminous efficiency can be useful to obtain a brighter lamp.
What is lamp?Lamp, lighting device, initially a jar housing a flame soaked in flammable substance, and later such light-producing devices as gas and electrical lamps.
The light was developed at least 70,000 years ago. Originally, it was a sunken rock filled in moss or other absorbent substance, saturated in animal fat, and burned. The ways to obtain a brighter lamp are
Increasing LED brightness with a lampshade or reflection.
To boost brightness, use greater color temperature LED lights.
Choosing LED lights with high luminous efficiency.
With increased voltage, LED lights get brighter.
Therefore, in above ways we can get brighter lamp.
To learn more about lamp, here:
https://brainly.com/question/9498978
#SPJ1
A family pool holds 25,000 quarts of water. How many mL is this? Report your answer using scientific notation.
1. 064qt=1L
Answers
This quantity may be expressed in scientific notation as 6.25 x 106 milliliters.
You can convert the number of quarts to liters using the conversion factor in order to determine the number of milliliters in a family pool that contains 25,000 quarts of water. 0.25 liters are contained in 1 quart.
This means that 6,250 liters are equal to 25,000 quarts, or 25,000 * 0.25.
The conversion factor can then be used to convert liters to milliliters. Milliliters are equivalent to one liter. Accordingly, 6,250 liters are equivalent to 6,250 * 1,000, or 6,250,000 milliliters.
This quantity may be expressed in scientific notation as 6.25 x 106 milliliters.
It is frequently used to convey extremely big or extremely small values. In this instance, scientific notation enables us to express the volume of the family pool in a more condensed and readable manner.
To learn more about Measurements,
https://brainly.com/question/27122947
#SPJ4
Calculate the standard potential, ∘, for this reaction from its equilibrium constant at 298 K. X(s)+Y3+(aq)↽−−⇀X3+(aq)+Y(s)=6.90×10−8 X ( s ) + Y 3 + ( aq ) ↽ − − ⇀ X 3 + ( aq ) + Y ( s ) K = 6.90 × 10 − 8
Answers
Answer: The standard potential is -0.141 V
Explanation:
To calculate the Gibbs free energy for given value of equilibrium constant we use the relation:
\(\Delta G=-RTlnK\)
where,
= standard Gibbs free energy = ?
R = Gas constant = 8.314 J/Kmol
T = temperature = 298 K
K = equilibrium constant =
Putting values in above equation, we get:
\(\Delta G=40853J\)
Also \(\Delta G=-nFE^0\)
where n = no of electrons gained or lost = 3
F = Faradays constant = 96500 C
\(E^0\) = standard potential = ?
\(40853=3\times 96500\times E^0\)
\(E^0=-0.141V\)
Thus the standard potential is -0.141 V
suppose a hydrogen atom in the ground state absorbs a 10.7 nm photon. how much kinetic energy will the emitted electron have when it is far away from the nucleus?
Answers
Answer: cheting is not alllowed is this homework or not
Explanation:
Which of the following elements has the ability to undergo sublimation
iodine
oxygen
carbon sodium
Answers
Answer:
iodine
Explanation:
Sandra reacts 3.345 g of zinc with hydrochloric acid. She obtained 3.956 g of zinc chloride. Calculate the percent composition of chloride in zinc chloride.
Answers
Explanation:
One molecule of the antibiotic known as penicillin G has a mass of ... b) If you isolate 115 g of zinc chloride, what is the percent yield of the metal ... This drives off the volatile hydrochloric acid, but the.
As Sandra reacts to the 3.345 g of zinc with hydrochloride acid as HCL the obtained value is about 3.956 g of zinc and the chloride the calculated value is.
The composition of chloride and zinc chloride is formed when a halogen gains an electron and when the Hydrogen chloride is dissolved.one molecule of the antibiotics known the penicillin i.e G has a mass of 115 g of zinc chloride, what is the percent yield of the metal This drives the volatile HCL acid.Learn more about the Sandra.
brainly.com/question/19793109.
There are 7.4 moles of Hydrogen gas in a container. How many molecules of H2 gas does the container hold?
Answers
7.4x6.022x10^23
Draw the best Lewis structure for XeI2.
Answers
In the XeI₂ Lewis structure, a xenon atom displays a xenon atom in the center, surrounded by two iodine atoms. There are two single bonds connecting the xenon atom to each iodine atom. There are three lone pairs on the xenon atom and both iodine atoms.
Lewis's structure is a pattern or diagram that describes the number of valence electrons of the atoms that will form chemical bonds. The Lewis structure is also known as the electron dot formula.
Several stages of how to write a Lewis structure:
First determine the center of the atom.Count on all the valence electrons of the atom. If the species is an ion, then add as many electrons as the ion has a negative charge or subtract the number of electrons with a positive charge.Create an electron pair for each bond.Complete the duplet or octet rule for the atoms bonded to the central atom.Add if necessary, the electron pairs on the central atom.If the atom center has not reached the octet rule, then a double bond must be formed to reach the octet rule.Learn more about Lewis's structure at https://brainly.com/question/20300458
#SPJ4

what product would be formed from the reaction of benzoyl chloride with aqueous naoh?
Answers
The product that would be formed from the reaction of benzoyl chloride with aqueous NaOH is benzoic acid (C₆H₅COOH).
Benzoyl chloride (C₆H₅COCl) reacts with NaOH (aqueous) to form benzoic acid (C₆H₅COOH), NaCl, and water (H2O). Benzoyl chloride is treated with a base like sodium hydroxide (NaOH) to produce a carboxylic acid. The reaction is known as hydrolysis because water is added to the compound to break down the bonds and produce a new molecule.
The balanced chemical equation is:
C₆H₅COCl + NaOH → C₆H₅COOH + NaCl
Learn more about hydrolysis here: https://brainly.com/question/1403345
#SPJ11
In the chemical equation A + B ⇔ C + D, which of the chemicals would be termed the reactant(s)?
A) A only
B) B only
C) A and B
D) C and D
E) C only
Answers
Correct answer is A and B. The reactant(s) in the chemical equation A + B ⇔ C + D would be option C, A and B.
A chemical reaction's reactants are the substances that take part in it. A chemical reaction is the term used to describe how atoms, which are the basic building blocks of matter, rearrange themselves to create new combinations. Reactants are raw materials that react with one another.
In the chemical equation A + B ↔ C + D, the reactants are the chemicals that participate in the reaction to form the products. In this case, the reactants are A and B.
To know more about chemical equation visit:-
https://brainly.com/question/30087623
#SPJ11
i need help pleaseeee
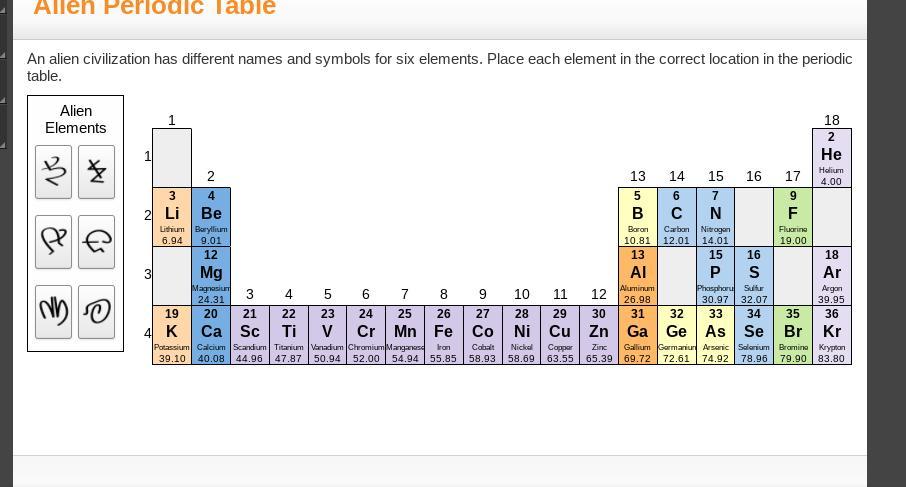
Answers
Answer:
1 is H
3 is NA
the one below 14 is SI
16 is O
the one below 17 is Ci
the one below 18 is NE
Explanation:
hope this helps!
Keq for H2 +I2 double sided arrow 2HI is 32.0 at equilibrium H2=I2=.400M what is the concentration of HI?
Answers
The concentration of HI is 25.6 M.
The equilibrium constant expression for the reaction H₂ + I₂ ⇌ 2HI is Keq = [HI]² / [H₂][I₂]. We are given that Keq = 32.0 at equilibrium, and that the initial concentrations of H₂ and I₂ are both 0.400 M. Let x be the equilibrium concentration of HI.
Using the equilibrium constant expression and the given values, we can set up the following equation:
32.0 = x² / (0.400)(0.400)
Solving for x, we get:
x = √(32.0 x 0.400 x 0.400)
x = 25.6 M
Therefore, the concentration of HI at equilibrium is 25.6 M.
To learn more about concentration, here
https://brainly.com/question/10725862
#SPJ1
Magnetic fields all have the same or equal strength .
true or false ?
Answers
How many liters (L) of water need to be added to prepare 4800mL of 0.5M NaCl from a 3M NaCl stock solution? (Use the formula M1V1=M2V2)
Answers
Answer: There is 0.8 liters (L) of water required to be added to prepare 4800mL of 0.5M NaCl from a 3M NaCl stock solution.
Explanation:
Given: \(M_{1}\) = 0.5 M
\(V_{1}\) = 4800 mL
Convert mL into L as follows.
\(1 mL = 0.001 L\\4800 mL = 4800 mL \times \frac{0.001 L}{1 mL}\\= 4.8 L\)
\(M_{2}\) = 3 M
Formula used to calculate the volume of water required as follows.
\(M_{1}V_{1} = M_{2}V_{2}\)
Substitute the values into above formula.
\(M_{1}V_{1} = M_{2}V_{2}\\0.5 M \times 4.8 L = 3 M \times V_{2}\\V_{2} = \frac{0.5 M \times 4.8 L}{3 M}\\= 0.8 L\)
Thus, we can conclude that 0.8 liters (L) of water need to be added to prepare 4800mL of 0.5M NaCl from a 3M NaCl stock solution.
How does an increase in temperature affect the collisions of gas particles with the walls of the container.
Answers
The average linear acceleration of an air changes with increase, which in turn causes the rms velocity (and average speed) of gas molecules to increase. As a result, the molecules hit their enclosures' walls .
What is collision and its types?Two items collide when they briefly come into contact with one another. To put it another way, a collision is a brief reciprocal encounter between two masses in which the velocity and energy of the masses change.
What chemical processes are brought on by collisions?According to the collision theory, for a physical reaction to take place, the interacting particles must come into contact with one another. The interaction forces affects the reaction's rate. Additionally, according to the hypothesis, reacting particles frequently collide without responding.
To know more about Collisions visit:
https://brainly.com/question/14881970
#SPJ4
What are the properties of covalent bonds and how do they differ from ionic bonds?
Answers
Answer:
Covalent bonds are formed by the sharing of electrons between two non-metallic atoms. These bonds are typically stronger and more stable than ionic bonds and are found in compounds such as water, hydrogen, and carbon dioxide. Covalent bonds differ from ionic bonds in that they do not involve the transfer of electrons from one atom to another.
Explanation:
Can someone help me?

Answers
Answer: 0.160 M
Explanation:

How can i solve this formula

Answers
Answer:
K2 Cr2 O7 is the empirical formula
Explanation:
Assume we have 100 g of the compound, because it makes the number of grams equal to the percentage.
Then convert grams of each element into moles
Then divide the moles of each element by the smallest number of moles attained to get the whole number mole ratios of the empirical formula
However, if you don't get whole numbers when you divide by the smallest number of moles, then multiply all moles by one common factor that makes them all whole numbers.
about how many days does it take to go from one moon phase to another
Answers
Answer:
After one month, the Moon returns to the same location in the sky as the Sun and we see a New Moon again. The demo represents a month as 28 days for simplicity. But the real cycle of phases takes 29.5 days to complete.
The outer energy level of a noble gas is
Select one:
a. empty.
b. filled.
c. half-filled.
Answers
What does the arrow itself mean?
Answers
Answer:
Explanation:
the arrow indicates a chemical reaction has occurred mean that
3. What is the greatest amount of H2O that can be made with 3.8 moles of H and 5 moles of
O? Which is the limiting reactant? Which reactant is in excess, and how many moles of it
are left over?
Answers
Answer: Hello!
first i believe we need a balanced equation to start...
i got 2H2 + 1O2 = 2H2O
This tells us that we need 2 moles of H2 for every 1 mole of O2 Since we only have 1 mole of H2 compared to the 5 moles of O2 hydrogen is the limiting reagent. For illustration, divide the balanced equation by 2 in order to get 1 mole of H2 If we start with 1.0 moles of H2 we'll produce 1.0 mole of H2O
Your welcome <3
Explanation:
The change in free energy of a chemical reaction represents
Answers
The change in free energy of a chemical reaction represents the amount of energy that is available to do useful work. It is a thermodynamic quantity that indicates the direction and extent of a chemical reaction.
If the free energy change (ΔG) is negative, the reaction is exergonic, meaning that energy is released and the reaction is spontaneous. This indicates that the products are more stable than the reactants, and the reaction will proceed in the forward direction. Conversely, if the free energy change (ΔG) is positive, the reaction is endergonic, meaning that energy is required for the reaction to occur and it is non-spontaneous. This indicates that the reactants are more stable than the products, and the reaction will proceed in the reverse direction. If the free energy change (ΔG) is zero, the reaction is at equilibrium, meaning that the forward and reverse reactions are occurring at equal rates and the concentrations of reactants and products do not change over time.
Learn more about free energy here:
https://brainly.com/question/15319033
#SPJ4
Answer in detail?? What precautions would you take to protect yourself from an earthquake, when you are at home and when you are outdoors?
Answers
Answer:
If you're indoors, stay inside. If you're outside, stay outside. If you're indoors, stand against a wall near the center of the building, stand in a doorway, or crawl under heavy furniture (a desk or table). Stay away from windows and outside doors.
Answer
Protect your head and neck with a large book, a pillow, or your arms. The goal is to prevent injuries from falling down or from objects that might fall or be thrown at you.If you are able, seek shelter under a sturdy table or desk. Stay away from outer walls, windows, fireplaces, and hanging objects.If you are unable to move from a bed or chair, protect yourself from falling objects by covering up with blankets and pillows.If you are outside, go to an open area away from trees, telephone poles, and buildings, and stay there.100 cm³ of a gas at 27°C is cooled to 20°C at constant pressure .Calculate the volume of gas at 20°C.
Answers
According to Charle's law, the volume of the given mass of a gas is directly proportional to its absolute temperature provided that the pressure is constant. Mathemically;
\(\begin{gathered} V\alpha T \\ V=kT \\ k=\frac{V}{T} \\ k=\frac{V_1}{T_1}=\frac{V_2}{T_2} \end{gathered}\)where;
V1 and V2 are the initial and final volume of the gas
T1 and T2 are the initial and final temperatures of the gas (in Kelvin)
Given the following parameters:
\(\begin{gathered} V_1=100\operatorname{cm}^3 \\ T_1=27^0C=27+273=300K \\ T_2=20^0C=20+273=293K \\ V_2=\text{?} \end{gathered}\)Substitute the given parameters into the formula;
\(\begin{gathered} V_2=\frac{V_1T_2}{T_1}^{} \\ V_2=\frac{100\times293}{300} \\ V_2=\frac{29300}{300} \\ V_2=\frac{293}{3} \\ V_2=97.67\operatorname{cm}^3 \end{gathered}\)Therefore the volume of the gas at 20°C is approximately 97.67cm³