An engineer examining the oxidation of SO₂ in the manufacture of sulfuric acid determines that Kc = 1.7x10⁸ at 600. K:2SO₂(g) + O₂(g) ⇄ 2SO₃(g)(a) At equilibrium, PSO₃ = 300. atm and PO₂ = 100. atm. Calculate PSO₂..
Answers
Nitric oxide (NO) is the catalyst used for the oxidation of SO2 to SO3 in the lead chamber process for the manufacture of sulphuric acid.
Nitric oxideThe volume of flask =10 liter
(a) 2SO2+O2⇌2SO3K=100
At an x a
end a/10 x/10 a/10
const KC=(x/10)(a/10)2(a/10)2=100
1001=10x⇒x=1/10
0.1 moles of O2 are present .
(b) 2SO2+O2⇌2SO3
At an x 2a
eq a/10 x/10 2a/10
contact
KC=(x/10)(a/10)2(102a)2⇒x/104=100
x=4/10=0.4 moles of O2.
To learn more about Nitric oxide visit the link
https://brainly.com/question/17092405
#SPJ4
Related Questions
!Urgent!
How does the law of conservation of mass apply to this reaction: Mg + HCl → H2 + MgCl2?
A. Hydrogen and chlorine need to be balanced. There is an equal amount of magnesium on each side
B. The equation needs to be balanced. There are fewer hydrogen atoms in the equation than magnesium or chlorine.
C. Only the hydrogen needs to be balanced. There are equal numbers of magnesium and chlorine.
D. The law of conservation of mass has already been applied. There is an equal number of each element on both sides of the equation.
Answers
This question was already answered here's the link
https://brainly.com/question/2023746
Why is copper a good conductor of electricity?
A.
Copper atoms hold onto their electrons very tightly.
B.
Electrons can move easily between copper atoms.
C.
Copper atoms are bonded together in molecules.
D.
Copper atoms can flow quickly along a wire.
Answers
Answer:
B. Electrons can move easily between copper atoms.
Explanation:
Use bond enthalpies to estimate the enthalpy change for each of the following reactions.
H2C=O(g)+HCl(g)→H3C−O−Cl(g)
H2O2(g)+2CO(g)→H2(g)+2CO2(g)
3H2C=CH2(g)→C6H12(g)
Answers
The enthalpy changes for each of the following reactions are:
H2C=O(g)+HCl(g)→H3C−O−Cl(g) = 256kJH2O2(g)+2CO(g)→H2(g)+2CO2(g) = 636kJ3H2C=CH2(g)→C6H12(g) = -164kJThe difference between total reactant and total product molar enthalpies estimated for substances in their standard states is the standard enthalpy of reaction for a chemical process. The quantity of heat evolved or absorbed in a process under constant pressure is referred to as enthalpy change. It is assigned the symbol ΔH, interpreted as "delta H".
Enthalpy is the product of internal energy and pressure multiplied by volume. We can't measure a system's enthalpy, but we can look at variations in enthalpy. The sum of internal energy (U) and the product of pressure (P) and volume (V) equals enthalpy (H) (V). The system's heat added or lost is quantified as a change in enthalpy (H), not the actual amount of heat.
To learn more about enthalpy change, here
https://brainly.com/question/29556033
#SPJ4
3. What property of metals makes them good conductors of heat?
Answers
Answer:
the electrons in them can move around easily
Explanation:
Answer:
The electrons in them being able to move around easily, and them being able to carry heat from one part to another.
What is the reactive intermediate in the reaction of 1,3-diene with hbr, resulting in 1,4-addition?
Answers
Allylic carbocation is the reactive intermediate in the reaction of 1,3-diene with hbr, resulting in 1,4-addition.
Are allylic carbocations more stable than tertiary?While stabilized primary resonance carbocations are less stable than tertiary carbocations( allyl cation, benzyl cation, and methoxymethyl cation), stabilized secondary resonance carbocations are more stable than tertiary carbocations.What's the structure of allylic?An allyl group is a substituent with the structural formula H2C = CH − CH2R, where R is the rest of the patch. It consists of a methylene ground( − CH2 −) attached to a vinyl group( − CH = CH2). The name is deduced from the Latin word for garlic, Allium sativum.Learn more about Allylic carbocation here:
https://brainly.com/question/28250336
#SPJ4
What is the control in the experiment?

Answers
Answer:
C. the amount of drug x given to mice
An aluminum ion has 13 protons, 14 neutrons, and 10 electrons. What is the charge of the aluminum ion?
Answers
Answer:
the charge of the aluminum ion is +3
HELP
Determine the empirical formula of a compound containing 48.38
grams of carbon, 6.74 grams of hydrogen, and 53.5 grams of oxygen.
In an experiment, the molar mass of the compound was determined to be 180.15 g/mol.
What is the molecular formula of the compound?
For both questions, show your work or explain how you determined the formulas by
giving specific values used in calculations.
Answers
Answer:
I recently answered this question. The response I submitted is included below. I beleive my answer should be correct.
Explanation:
Question 1:
C: 48.38g(1mol/12g) = 4.0317
H: 8.12g(1mol/1.01g) = 8.12
O: 53.5g(1mol/16g) = 3.34375
Divide by the smallest amount (3.34375)
C = 4.0317/3.34375 = 1.206 = 1
H = 8.12/3.34375 = 2.42 = 2
O = 3.34375/3.34375 = 1
Empirical formula = CH2O
Question 2:
Molecular formula = n(empirical formula)
n = molar mass (compound)/molar mass (empirical)
Empirical formula: CH2O
Molar mass of CH2O = 12 + 2x1 + 16 = 30 g/mol
Molar mass of compound: 180.15 g/mol
\(n = \frac{180.15g/mol}{30g/mol} = 6\)
Molecular formula = C6H12O6
my teacher hasnt graded yet, but i got the same answer as the guy above. i did my best to explain the process for y'all if you genuinely don't understand/know how to do it. THE PROCESS IS VERY LONG, but youll get it eventually if i didnt help lol GOODLUCK
and yes, the molecular formula is C6H12O6



If the solution is 400 ml and solvent is 300ml so what is concentration of solution
Answers
Answer:
Explanation:
Explanation:
Solute = solution - solvent
Solute = 400 - 300
Solute = 100ml
Percentage of Solute = volume of Solute / volume of solution
= 25 %
Hope it helps you.
Câu 36: _VD _Hình lập phương có thể tích là 1253 thì diện tích đáy là:
A. cm2 B. 5cm2 C. 25cm2 D. 52cm2
Answers
Answer:
c
Explanation:
Cạnh của hình lập phương là
³\(125 = 5cm
Diện tích đáy của hình lập phương là
5²=25cm²
Đáp án C
The following compounds will
decompose on heating except
A Ag2CO3.
B. CaCO3.
C. K2CO3
D. PbCO.
E. ZnCO3
Answers
AnsThe option C is correct
How many atoms of carbon are present in 1.0g of CH3CH2OH?
Answers
A compound with a mass of 48.72g is found to contain 32.69g of zinc and 16.03g of sulfur. What is the percentage composition of the compound
Answers
Answer:
dic
Explanation:
I do believe i need help.....


Answers
Answer:
C,D, and A I believe
Explanation:
why is OH on the outside of the lewis structure for methanol?
Answers
In the Lewis structure of methanol (CH3OH), the OH group is placed on the outside because it is an important functional group that influences the chemical properties and reactivity of the molecule.
The Lewis structure is a representation of a molecule that shows the arrangement of atoms and valence electrons. In methanol, carbon (C) is the central atom bonded to three hydrogen (H) atoms and one oxygen (O) atom. The oxygen atom forms a single bond with carbon and also has two lone pairs of electrons.
The placement of the OH group (hydroxyl group) on the outside of the Lewis structure is significant because it determines the chemical behavior of methanol. The OH group consists of an oxygen atom bonded to a hydrogen atom and represents the presence of an alcohol functional group.
In organic chemistry, functional groups are specific arrangements of atoms within a molecule that give rise to characteristic chemical reactions and properties. The presence and position of functional groups can greatly influence the behavior and reactivity of a compound. In the case of methanol, the hydroxyl group provides the molecule with its characteristic properties.
know more about valence electrons here:
https://brainly.com/question/371590
#SPJ8
How many moles of helium are needed to fill a balloon to a volume of 5.3 L at 22 ℃ and 632 mmHg?
Answers
Answer:
0.18 moles
Explanation:
Applying,
PV = nRT................... Equation 1
Where P = pressure, V = volume, n = number of moles, R = molar gas constant, T = temperature.
make n the subject of the equation
n = PV/RT............... Equation 2
Given: V = 5.3 L, T = 22 °C = (22+272) K = 295 K, P = 632 mmHg = (0.00131579×632) = 0.8316 atm, R = 0.083 L.atm/K.mol
Substitute these values into equation 2
n = (0.8316×5.3)/(0.083×295)
n = 0.18 moles
Dissolved: Will give brainliest

Answers
Answer:
im 99% sure it's 175.
Explanation:
keep trucking, i know school is hard but you're doing amazing
How the Bohr model explains both of these observations
Answers
The Bohr model explains the observations by suggesting that electrons exist in specific energy levels and transitions between these levels cause the observed colors.
The Bohr model of an atom explains the observations of line spectra and quantized energy levels. Line spectra is a phenomenon where atoms emit or absorb light at specific wavelengths. Quantized energy levels refer to the specific energies that electrons can possess while occupying specific energy levels.
The Bohr model explains both of these observations by proposing that electrons can only exist in specific energy levels and can move between them by absorbing or emitting photons of specific energies. An electron in an atom can exist only in one of the allowed energy levels.
These energy levels are defined by the Bohr radius formula:
\(r(n) = n^2 * h^2 / 4\)π\(^2mke^2\)
Where r(n) is the radius of the nth energy level, n is an integer representing the energy level, h is Planck's constant, m is the mass of the electron, ke is Coulomb's constant, and e is the charge of the electron.Electrons emit light when they move from a higher energy level to a lower one and absorb light when they move from a lower energy level to a higher one.
The energy of the photon emitted or absorbed is equal to the difference in energy between the two levels. This explains why line spectra occur, as each atom emits or absorbs light at specific wavelengths corresponding to the energy difference between its allowed energy levels.The Bohr model's proposal of quantized energy levels provides an explanation for the stability of atoms. Electrons in an atom can't exist between energy levels, so they can't radiate energy and spiral into the nucleus.
Know more about Bohr model here:
https://brainly.com/question/29400473
#SPJ8
Calculate the volume in L of 11.6 moles of Neon at 120 K when it has a pressure of 25.9 atm
Answers
Answer:
The volume of the gas is approximately 4.41 liters
Explanation:
The details of the data of the Neon gas are;
The number of moles of Neon gas present, n = 11.6 moles
The temperature of the sample of Neon gas, T = 120 K
The pressure of the sample of the Neon gas, P = 25.6 atm
By the ideal gas equation, we have;
P·V = n·R·T
Where;
R = The universal gal constant = 0.08205 L·atm·mol⁻¹·K⁻¹
Therefore, we get;
V = n·R·T/P
Which gives;
V = 11.6 moles × 0.08205 L·atm·mol⁻¹·K⁻¹ × 120 K/(25.9 atm) ≈ 4.4097915 L
The volume of the gas, V ≈ 4.41 L.
Which of the following is always equal to the number of protons in an atom 1pc
of a given element?
Mass number
Olonic number
O Atomic number
Answers
Answer:
\(\huge\mathfrak\purple{answer..} \\ \\ \huge\mathfrak\green{atomic \: number} \\ \\ \huge\mathfrak\red{hope \: it \: helps...}\)
OMG PLEASE HELP IM DOING SO WELL IN SCIENCE AND THIS IS GRADED AND MY PROFEESER WONT HELP
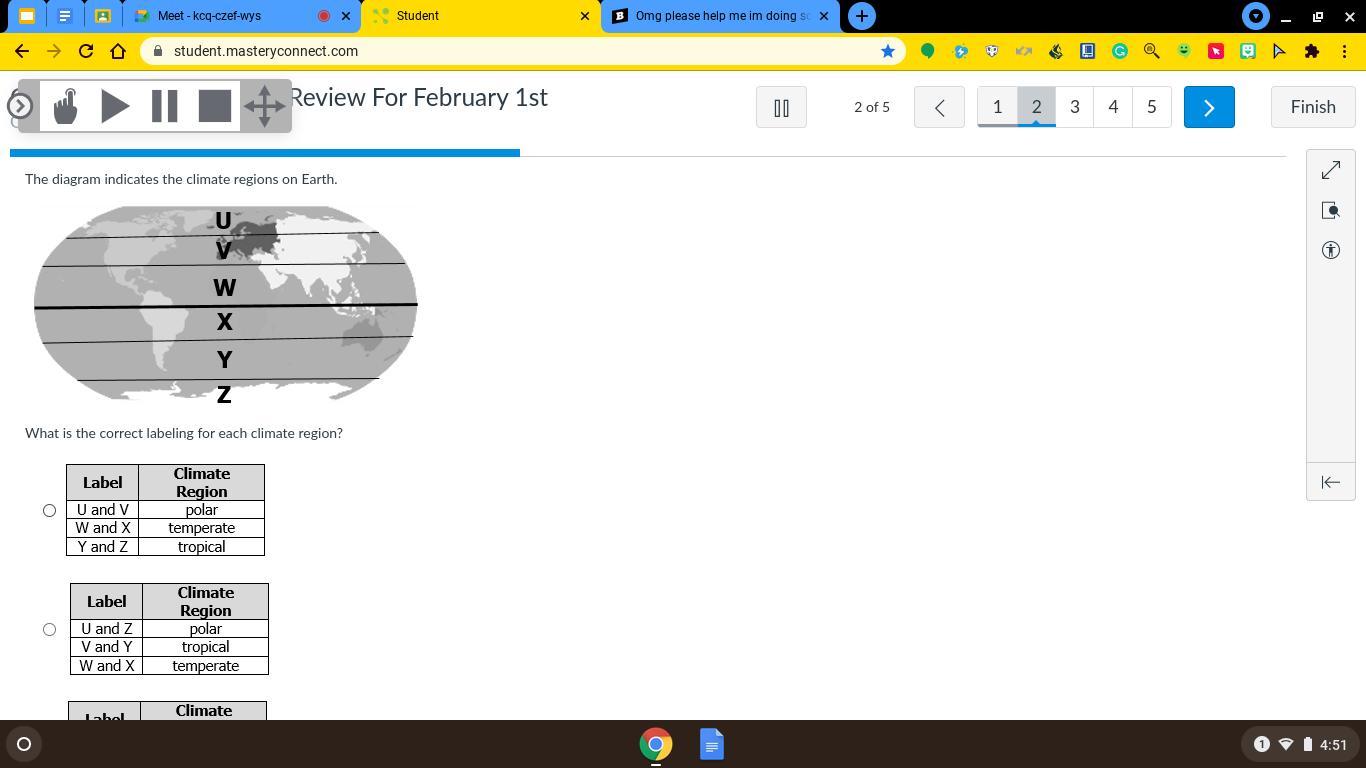

Answers
Answer:
bbbbbbbbbbbbbbbbbbb
Explanation:
Classify each substance as a pure substance or a mixture. If it is a pure substance, classify it as an element or a compound. If it is a mixture, classify it as homogeneous or heterogeneous.
a. wine
b. beef stew
c. iron
d. carbon monoxide

Answers
What would heating a sealed glass jar do?
O A. Increase the size of the gas molecules
B. Increase the number of gas molecules
C. Increase the pressure of the gas inside
D. Increase the volume of the gas inside
Answers
The right response is to raise the internal gas pressure. When a sealed glass jar is heated, the temperature of the gas within rises, causing the gas molecules to travel faster and collide with the jar's walls more often and with more force, increasing the gas's pressure.
What is gas molecule?Gas molecules are made up of atoms that are linked together. These interatomic connections function similarly to springs, connecting atoms of varying masses. This connection vibrates at a constant frequency known as the natural frequency. Atoms, molecules, and/or ions are all present in gases, liquids, and solids, but their behavior varies between the three phases. The graphic below depicts the tiny differences. A gas as seen via a microscope. A gas is a condition of stuff that does not have a set volume or form. Air, water vapor, and helium are examples of gases. A gas is a kind of stuff that does not have a defined volume or shape. To put it another way, a gas takes on the shape and volume of its container.
Here,
The correct answer is Increase the internal gas pressure as When a sealed glass jar is heated, the temperature of the gas within rises, forcing the gas molecules to travel faster and hit with the jar's walls more often and with more force, raising the gas's pressure.
To know more about gas molecule,
https://brainly.com/question/29848914
#SPJ1
the knives used in gks can: i. generate free radicals. ii. excite electrons to higher energy levels. iii. eject electrons from molecular orbitals.
Answers
The correct option is C, Knives are made from materials which includes metallic, which do now not generally generate unfastened radicals, and eject electrons from molecular orbitals.
Molecular orbitals are regions of space round a molecule wherein the possibility of finding an electron is excessive. they may be shaped by using the combination of atomic orbitals, which can be areas of space round an atom where the chance of finding an electron is high. whilst two or more atomic orbitals overlap, they integrate to shape molecular orbitals.
There are forms of molecular orbitals: bonding and antibonding. Bonding molecular orbitals result from positive interference of the atomic orbitals, whilst antibonding molecular orbitals result from damaging interference. Electrons in bonding molecular orbitals assist to maintain the atoms together in a molecule, at the same time as electrons in antibonding molecular orbitals weaken the bond.
To learn more about Molecular orbitals visit here:
brainly.com/question/30907237
#SPJ4
A galaxy is a system of celestial bodies, such as stars, planets, several solar systems, and interstellar gas and dust. Our solar system is a part of a galaxy called
Answers
Answer:
The Milky Way
Explanation:
Answer:
The Milky Way
Explanation:
Example 11.1âA High-Performance Bicycle Tire Has a Pressure of 125 psi. What Is the Pressure in mmHg?
Answers
The pressure of the high-performance bicycle tire in mmHg is 6464.375.
The pressure of the high-performance bicycle tire is 125 psi, which stands for pounds per square inch.
To convert psi to mmHg, we need to use the conversion factor 1 psi = 51.715 mmHg.
Therefore, to find the pressure in mmHg, we can simply multiply the psi value by this conversion factor:
125 psi x 51.715 mmHg/psi = 6464.375 mmHg
It's important to note that pressure is a crucial factor in the performance of tires, as it affects their grip, rolling
resistance, and overall stability.
Maintaining the proper pressure can not only improve performance but also prevent premature wear and tear, as well
as potential safety issues.
To learn more about pressure click here https://brainly.com/question/18431008
#SPJ11
what is the mass in grams of potassium chloride contained in 430.ml of a .193m potassium chloride solution
Answers
The mass in grams of potassium chloride in 430 ml of a .193 m potassium chloride solution is 14.4 grams. Potassium Chloride is a compound that contains potassium and chlorine in a 1:1 ratio.
The mass in grams of potassium chloride contained in 430 ml of a .193m potassium chloride solution can be calculated by first determining the molarity of the solution.
Molarity = moles of solute / volume of solution in liters. The solution's molarity is 0.193 mol/L because it is given in the problem statement.
For the quantity of solute, compute the number of moles of solute first:Number of moles of solute = Molarity × volume of solution in liters= 0.193 mol/L × 0.43 L= 0.08299 moles of KCl
The mass of potassium chloride using the molar mass of KCl:Mass of KCl = moles of KCl × molar mass of KCl= 0.08299 moles × 74.55 g/mol (molar mass of KCl)= 6.1819 g = 6.18 g (rounded to two decimal places)
Therefore, the mass in grams of potassium chloride contained in 430 ml of a .193m potassium chloride solution is 14.4 grams.
to know more about potassium chloride refer here:
https://brainly.com/question/22528097#
#SPJ11
A 4 feet tall student went summing pool. He saw depth of water in pool less than 4 feet.Will he drowned.Write reason
Answers
Answer:
Question says , the height of the student = 4 feet, ... This means, if the student goes for swimming in the pool, however he does not know swimming, he will not be drowned until he is suffering from an injury or external force.
Magnesium absorption is enhanced by the presence of:.
Answers
Magnesium absorption is enhanced by the presence of vitamin D and calcium.
The absorption of magnesium can be improved by dietary and supplemental factors. Vitamin D and calcium both aid in magnesium absorption and play essential roles in bone health.Calcium and magnesium both work together for bone health. Magnesium is also necessary for the absorption and metabolism of calcium. Vitamin D is essential for calcium absorption and utilization, so magnesium's absorption is also aided by this vitamin. Other nutrients that help with magnesium absorption include vitamin B6, vitamin C, and potassium.
In summary, Vitamin D and calcium are the factors that enhance magnesium absorption in the human body. Along with these two, Vitamin B6, Vitamin C, and Potassium also help in the absorption of magnesium.
To know more about magnesium, click here
https://brainly.com/question/8351050
#SPJ11
Help me about this please

Answers
1A.matter can neither be created nor be destroyed
6.balencing equation steps:--
balencing other than hydrogen nd oxygen
balencing oxygen
balencing hydrogen
balencing charge