Answers
Answer: Energy = 6.0236 X 10 ^-19J per atom and 362.80KJ/mol
Explanation:
The energy of a photon is given by:
E = hf
but f =c/ λ
therefore E= = hc/ λ
Where h is the Planck constant, 6.626x10^-34 J*s
c = speed of light = 3.0 x 10 ^ 8 m/s
λ n= wavelength = 330nm
but 10^9 nm = 1m
330nm= 3.30 x 10^-7m
E= 6.626 X 10 ^-34Js X3.0 x 10 ^ 8 m/s / 3.30 x 10^-7m
= 6.0236 X 10 ^-19J per atom ---Which is energy of a single photon,
Now 1 mole = 6.022x10^23 units, (Avogadro number)
therefore , the energy per 1 mole of photons is
6.0236 x 10^-19 J/atm X 6.023 X 10 ^23 atm/ 1 mol = 362,803.618J/mol
changing to KJ/mol becomes 362,803.618/ 1000= 362.80KJ/mol
Related Questions
Which of the following elements would be the most stable (unreactive)?
1 ) Fr
2) Fe
3) H
4) Ne
Answers
4) Ne
General Formulas and Concepts:Chemistry
Atomic Structure
Reading a Periodic TableExplanation:The most stable (unreactive) elements are found in group 18. They are known as the Noble Gases.
Noble Gases are inert, meaning they do not react easily with other elements. The reason is because their valence electron shells are filled.
Therefore, our best answer is 4) Ne.
Answer:
\(\boxed {\boxed {\sf 4. \ Ne }}\)
Explanation:
On the Periodic Table, the last group of elements are the Noble Gases. These are the most unreactive or stable elements.
According to the Octet Rule, atoms always want to have 8 valence electrons. The Noble Gases already satisfy this rule and have full outer shells, therefore they don't react with other elements often.
The Noble Gases are helium (He) , neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og).
The only answer choice that is a Noble Gas is 4. Ne
NEED HELP ASAP
Question 7 (5 points)
(05.05 MC)
A synthesis reaction takes place when carbon monoxide (CO) and hydrogen gas (H2) react to form methanol (CH3OH). How many grams of
methanol are produced when 7.0 grams of carbon monoxide reacts with excess hydrogen gas? (5 points)
20 grams
7.0 grams
8.0 grams
6.1 grams
Answers
Explanation:
Write the balance equation for the reaction taking place:
CO(g) + 2H2(g) ==> CH3OH ... balanced equation
Find the limiting reactant:
moles CO present = 14 g x 1 mol CO / 28 g = 0.5 moles COmoles H2 present = 1.5 g x 2 mol H2 / 2 g = 0.75 moles H2OBecause according to the balanced equation it takes 2 moles H2 for every 1 mole CO, there isn't enough H2, so it is limiting and will dictate how much CH3OH can be made.
Mass of CH3OH produced = 0.75 moles H2 x 1 mol CH3OH / 2 mol H2 x 32 g CH3OH / mol = 12 g CH3OH
A student was instructed to measure 30 mL of water in a 50-mL beaker. She recorded the following data in her notebook:
Mass of water 30.0 g
Density of water at Room Temperature 0.997 g/mL
Calculate the student's absolute error.
Answers
The student's absolute error, given the data from the question is 0.09 mL
How to determine the volume the student measuredWe'll begin by obtainig the volume of the water the student measured. This can be obtained as follow:
Mass of water = 30.0 gDensity of water = 0.997 g/mLVolume of water =?Density = mass / volume
Thus,
Volume = mass / density
Volume of water = 30 / 0.997
Volume of water = 30.09 mL
How to determine the absolute errorThe absolute error can be obatined as illustrated below:
Accepetd value = 30 mLMeasured value = 30.09 mLAbsolute error =?Absolute error = Measured value - accepted value
Absolute error = 30.09 - 30
Absolute error = 0.09 mL
Thue, the student's absolute error is 0.09 mL
Learn more about density and absolute error:
https://brainly.com/question/952755
https://brainly.com/question/11599357
#SPJ1
1. A rocket can be powered by the given reaction: N₂O4(1)+2N2H4(1)→ 3N2(g) + 4H₂O(g). An engineer designed the rocket to hold 1.00 kg of N₂O4 and excess N₂H4. [N 14.007 amu; H 1.008 amu; O 15.999 amu] (1000 g = 1kg) a) Given the balanced chemical equation above, what is the mole relationship between N₂O4 and N₂ ? (3 pts) N₂O +z M >N₂) 1/ b) How much N₂ would be produced with the 750 g N₂O4? (10 pts) c) If 650 g of N₂ was obtained, what is the percent yield?
Show work please
Answers
According to the balanced reaction, one mole of N₂O₄ produce 3 moles of N₂. Thus 750 g of N₂O₄will produce 684.7 g of N₂. If the actual yield of N₂ is 650 g, the percent yield is 94.9 %.
What is percent yield?The percent yield of a reaction is the ratio of actual yield to the theoretical yield multiplied by 100.
According to the given balanced reaction, one mole of N₂O₄ produce 3 moles of N₂. The molar mass of N₂O₄ is 92 g/mol and the molar mass of 3 moles of N₂ is 84 g. Thus 92 g of N₂O₄ produce 84 g of N₂.
The mass of N₂ produced from 750 g of N₂O₄ is then calculated as follows:
mass of N₂ = ( 750 × 84 /92)
= 684.7 g.
The actual yield of N₂ is given 650 g. Thus the percent yield of N₂ is calculated as:
Percent yield = (650 / 684.7) × 100
= 94.9 %.
Hence, the amount of N₂ produced from 750 g of N₂O₄ is 684.7 g and the percent yield is 94.9%.
To refer more about percent yield find the link here:
https://brainly.com/question/12704041
#SPJ1
How many alkyl substituents does N-ethyl-N-methylaniline have?
one
two
O three
o eight
none
Answers
Answer:
Two.
Explanation:
Hello!
In this case, considering the attached picture wherein you can see the presence of the parent chain as aniline, we can see radicals at the nitrogen, named by N-ethyl and N-methyl which are the present alkyl substituents. In such a way, the benzene ring is not considered an alkyl radical because it belongs to the parent chain on aniline and therefore, N-ethyl-N-methylaniline has two alkyl substituents.
Best regards!

There are two alkyl substituents attached to the nitrogen; one ethyl and one methyl group
Aniline consists of a benzene ring to which an -NH2 moiety is attached. Usually, it is possible for the any of the two hydrogen atoms in the -NH2 moiety to become substituted leading to N-alkylated product.
In the compound N-ethyl-N-methylaniline, there are two alkyl substituents attached to the nitrogen; one ethyl and one methyl group as shown in the image attached to this answer.
Learn more: https://brainly.com/question/6284546

Explain how a rainbow is produced
Answers
A rainbow is produced through a proces that includes refraction, reflection, and dispersion of sunlight.
What more should you know about the production of rainbows?A rainbow is formed when sulinght is refracted and reflected by rain drops in the atmospher.
The sunlight is split into its component colors, which is why rainbows appear as having an array of colors. This is due to each color being bent by a different amount during refraction.
The colors of a rainbow are always in the same order, with red on the outside and violet on the inside.
Find more exercises on rainbows;
https://brainly.com/question/7965811
#SPJ1
The following question was posed on an exam:
An unknown non-metal element (Q) forms two gaseous fluorides of unknown molecular formula. A 3.2g sample of Q reacts with fluorine to form 10.8 g of the unknown fluoride A. A 6.4 g sample of Q reacts with fluorine to form 29.2 g of unknown fluoride B. Using these data only, demonstrate by calculation and explanation that these unknown compounds obey the Low of Multiple Proportions.
A student responded with the following answer: The Law of Multiple Proportions states that when two elements form two or more compounds, the ratios of the masses of the elements between the two compounds are in o simple whole number ratio. So, looking of the data above, we see that the ratio of the moss of element Q in compound A to the mass of element Q in compound B is, which is a simple whole number ratio. This demonstrates that these compounds obey the low of Multiple Proportions. Assess the accuracy of the students answer. In your assessment, you must determine what information is correct or Incorrect, provide the correct information where needed, explain whether the reasoning is logical or not, and provide logical reasoning where needed.
Answers
Answer:
The answer given by the student is not totally correct.
Explanation:
Law of multiple proportions states that an element Q will react with different volume of Fluorine to produce two non-similar compounds. Hence, the ratio of the masses(Fluorine only) needs to be an absolute reduced value.
Given that:
3.2 g of a sample of Q reacts with Fluorine to form 10.8 g of the unknown fluoride A.
This means the mass of Fluorine present in the compound = 10.8 g - 3.2 g = 7.6 g
Thus, 3.2 g of a sample of Q reacts with 7.6 g of Fluorine.
Also, 6.4 g sample of Q reacts with Fluorine to form 29.2 g of unknown fluoride B.
If we divide the samples by (2), we have 3.2 g sample of Q reacting with Fluorine to form 14.6 g of unknown fluoride B.
This means the mass of Fluorine present in the compound = 14.6 g - 3.2 g = 11.4 g
Thus, 3.2 g Q reacted here with 11.4 g fluorine.
Hence, 11.4/7.6 = 1.5 = 3/2
This above therefore satisfies the law of multiple proportions.
This is not aligned with what the student did, what the student did was to relate the amount of Q used to make A and B. Suppose, we start with twice amount of Q, the ratio would have been smaller(i.e. the ratio of Q).
So, this doesn't relate to the law of multiple proportions.
The law of multiple proportions is specifically concerned with the mass of the element rather than Q that can react with Q. Therefore, there is more reason to relate the two samples of equal masses of Q that react with different masses of Fluorine. The ratios of Fluorine will then be small whole numbers.
The earth is farthest from the sun in
Answers
Answer:
July/the winter
Explanation:
Pls answer my question https://brainly.com/question/18950623
July, I just took the test
Which of the following is NOT an example of potential energy?
A. Gravitational pull C. Chemical Bonds
B. Nuclear energy D. Electricity
Answers
Answer:
Answer C
Explanation:
Chemical Bonds
The rotational spectrum of 79BrºF shows a series of equidistant lines spaced 0-714 33 cm - apart. Calculate the rotational constant B, and hence the moment of inertia and bond length of the molecule. Determine the wavenumber of the J = 9+= 10 transition, and find which transition gives rise to the most intense spectral line at room temperature (say 300 K).
and calculate the number of revolutions per second which the Brf molecule undergoes when in (a) the J = 0 state, (b) the J = 1 state, and (c) the J = 10 state. Hint: Use E = {lwin conjunction with Eqs (2.10) and (2.13), but remember that here w is in radians per second.[its Q season 2 from fundamentals of molcular spectruscopy . banwell.c.n]
Answers
In the J = 0 state, the BrF molecule does not undergo any revolutions per second. In the J = 1 state, it undergoes approximately 0.498 revolutions per second, and in the J = 10 state, it undergoes approximately 15.71 revolutions per second.
To calculate the rotational constant B, we can use the formula:
B = 1 / (2 * π * Δν)
Where:
B = rotational constant
Δν = spacing between consecutive lines in the rotational spectrum
Given that the spacing between consecutive lines is 0.71433 cm^(-1), we can substitute this value into the formula:
B = 1 / (2 * π * 0.71433 cm^(-1))
B ≈ 0.079 cm^(-1)
The moment of inertia (I) of the molecule can be calculated using the formula:
I = h / (8 * π^2 * B)
Where:
h = Planck's constant
Given that the value of Planck's constant (h) is approximately 6.626 x 10^(-34) J·s, we can substitute the values into the formula:
I = (6.626 x 10^(-34) J·s) / (8 * π^2 * 0.079 cm^(-1))
I ≈ 2.11 x 10^(-46) kg·m^2
The bond length (r) of the molecule can be determined using the formula:
r = sqrt((h / (4 * π^2 * μ * B)) - r_e^2)
Where:
μ = reduced mass of the molecule
r_e = equilibrium bond length
To calculate the wavenumber (ν) of the J = 9+ to J = 10 transition, we can use the formula:
ν = 2 * B * (J + 1)
Substituting J = 9 into the formula, we get:
ν = 2 * 0.079 cm^(-1) * (9 + 1)
ν ≈ 1.58 cm^(-1)
To determine the most intense spectral line at room temperature (300 K), we can use the Boltzmann distribution law. The intensity (I) of a spectral line is proportional to the population of the corresponding rotational level:
I ∝ exp(-E / (k * T))
Where:
E = energy difference between the levels
k = Boltzmann constant
T = temperature in Kelvin
At room temperature (300 K), the population distribution decreases rapidly with increasing energy difference. Therefore, the transition with the lowest energy difference will have the most intense spectral line. In this case, the transition from J = 0 to J = 1 will have the most intense spectral line.
To calculate the number of revolutions per second, we can use the formula:
ω = 2 * π * B * J
Where:
ω = angular frequency (in radians per second)
J = rotational quantum number
For J = 0:
ω = 2 * π * 0.079 cm^(-1) * 0 = 0 rad/s
For J = 1:
ω = 2 * π * 0.079 cm^(-1) * 1 ≈ 0.498 rad/s
For J = 10:
ω = 2 * π * 0.079 cm^(-1) * 10 ≈ 15.71 rad/s
For more such questiosn on BrF molecule visit;
https://brainly.com/question/30624940
#SPJ8
True or False: When you divide for density, you can put mass first or volume
first - the order doesn’t matter.
a. True b. False
Answers
Answer:
false
Explanation:
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
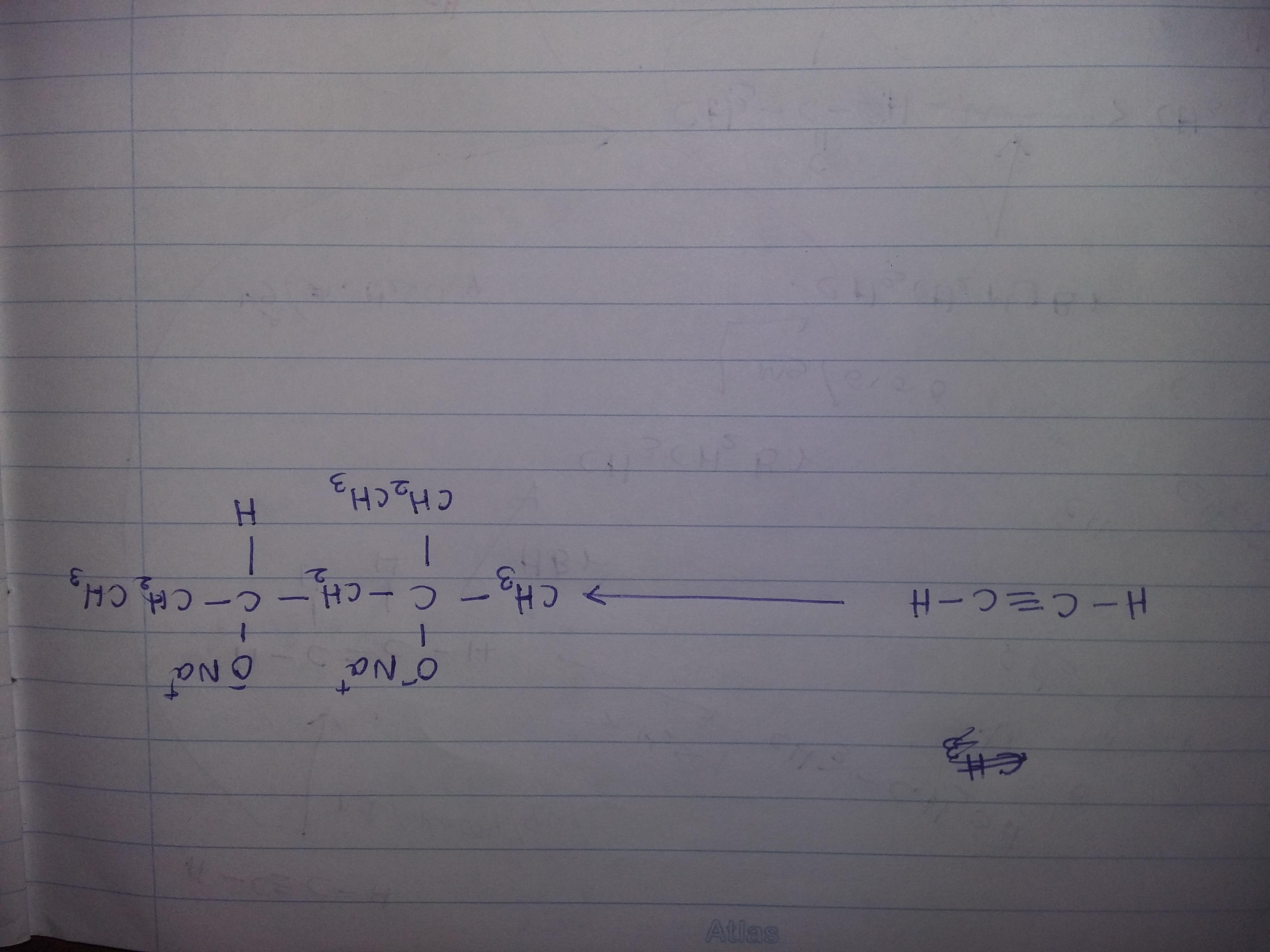
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
0.12g of compound "Y" dissolves in 10mlof acetone at 25 degree celsuis and 0.85g of the same compound dissolves in 10ml of boiling acetone. what volume of acetone would be required to purify a 5.0g sample of compound?
Answers
The volume of acetone would be required to purify a 5.0g sample of compound is 58.82ml
Volume calculation.
Let use the solubility data of acetone provided to calculate the volume.
First we will need to calculate the solubility of Y in 25 degree.
Solubility of Y= 0.12g/10ml= 0.012g/ml.
Solubility of y in boiling acetone = 0.85g/10ml =0.085g/ml.
In order to purify y, we need to dissolve the amount of y in the 5.0g sample.
The amount of acetone needed to dissolve y in the sample is
amount acetone= mass of y/ solubility of y in boiling acetone.
amount acetone= 5.0g/0.085g/ml= 58.82ml.
Therefore, the volume of acetone would be required to purify a 5.0g sample of compound is 58.82ml
Learn more about volume below.
https://brainly.com/question/30164274
#SPJ1
10. A small helium tank claims to be able to fill 30 balloons to a volume of 3.15 L
at a pressure of 101 kPa. How many liters of helium will the tank be able to
produce at a pressure of 94.2 kPa? Assume that the temperature of the tank
remains the same throughout.
Answers
Taking into account the Boyle's law, 3.377 L of helium the tank will be able to produce at a pressure of 94.2 kPa.
The gas laws are a set of chemical and physical laws that allow determining the behavior of gases in a closed system. The parameters evaluated in these laws are pressure, volume, temperature, and moles.
Boyle's law is one of the gas laws that relates the volume and pressure of a certain quantity of gas kept at constant temperature.
This law states that the pressure of a gas in a closed container is inversely proportional to the volume of the container, when the temperature is constant. That is, if the pressure increases, the volume decreases; while if the pressure decreases, the volume increases.
Mathematically, Boyle's law states that the product of pressure and volume is constant:
P×V= k
Studying two different states, an initial state 1 and a final state 2, it is satisfied:
P1× V1= P2×V2
In this case, you know:
P1= 101 kPa V1= 3.15 L P2= 94.2 kPa V2= ?Replacing in Boyle's law:
101 kPa× 3.15 L= 94.2 kPa× V2
Solving:
\(V2=\frac{101 kPa x3.15 L}{94.2 kPa}\)
V2= 3.377 L
In summary, 3.377 L of helium the tank will be able to produce at a pressure of 94.2 kPa.
Learn more:
https://brainly.com/question/4147359?referrer=searchResults
Complete question and balance answer. If anyone can help I’d appreciate it
Ag+N2
Ba(NO3)2
K2SO4+Ca(MnO4)2
CH4+O2
Mg(OH)2
S2H6+O2
Na2CrO4+ALPO4
SrS
NH4+CO3
Al(NO3)
Ba(NO3)+Fe
Cr+p (cr=2+)
GaN+KF
KBr
Li2O
C4H6+O2
HNO3+AL(OH)3
Mg(C2H3O2)2+K3BO3
Answers
2223 25
TIME REMAINING
01:47:22
21
What can the arrow in a chemical reaction be translated to mean? Check all that apply.
O yields
Oaccompanied by
Dreact to form
Dadded to
Dexcept
Answers
The arrow in a chemical reaction can be translated as the following:
A. yields
C. react to form
Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
A student places 12.8 mL of water in a graduated cylinder. The mass of the water and the graduated cylinder is found to be 46.304 grams, and then the balance is tared. After adding a number of metal pellets to the graduated cylinder, the liquid level in the cylinder is now 25.5 mL. The cylinder with the water and metal pellets is placed back on the balance, and the measured mass is 75.440 grams. Calculate the density of the metal pellets in g/mL.
Answers
The density of the metal palates is 2.29 g/mL.
What is density?The term density refers to the ratio of the mass to the volume of a body. We know that the volume of a solid can easily be obtained by the use of the displacement method in which water is ejected from the vessel.
Hence;
Mass of the metal pellets is = 75.440 grams - 46.304 grams= 29.136 grams
Volume of the water = 25.5 mL - 12.8 mL = 12.7 mL
Hence density = mass/volume = 29.136 grams/12.7 mL = 2.29 g/mL
Learn more about density:https://brainly.com/question/15164682
#SPJ1
Ionic compounds form because ___ charges attract
Answers
Answer:
Ionic bonding is the attraction between positively- and negatively-charged ions. These oppositely charged ions attract each other to form ionic networks (or lattices). Electrostatics explains why this happens: opposite charges attract and like charges repel.
Explanation:
Ionic compounds form because opposite charges attract.
In an ionic compound, positive and negative ions come together to form a stable structure. This occurs when an atom loses electrons to become a positively charged ion (cation) and another atom gains those electrons to become a negatively charged ion (anion). The positive and negative charges of the ions attract each other, resulting in the formation of an ionic bond.
This attraction between positive and negative charges is what holds ionic compounds together. The strong electrostatic forces of attraction between ions result in a lattice structure, where each ion is surrounded by oppositely charged ions.
Thus, ionic compounds are formed because opposite charges attract.
Learn more about ionic bond,here:
https://brainly.com/question/9075398
#SPJ6
The graph shows the altitude and temperature for different layers of Earth’s atmosphere. Based on the diagram, which of the following identifies a characteristic of the atmosphere between the stratopause and the tropopause?
answer choices
Cloud formation occurs in the stratosphere.
The warmest air is found in the mesosphere.
The ozone layer is in the stratosphere.
The troposphere is the closest layer to the sun.

Answers
The ozone layer is present in the stratosphere layer of the Earth's atmosphere. Therefore, option (C) is correct.
What is the atmosphere?An atmosphere can be defined as layers of gases that envelop a planet and is held in place due to the gravity of the planetary body. A planet contains an atmosphere when the gravity is great and the temperature of the atmosphere of any planet is low.
Earth's atmosphere is made of Nitrogen (78 %), Oxygen (21%), Argon (0.9%), and Carbon dioxide (0.04 %). The troposphere can be defined as the lowest layer of the atmosphere. The troposphere contains 75 to 80 % of the mass of the atmosphere.
The stratosphere contains the ozone layer, at an altitude between 15km and 35km. This atmospheric layer absorbs most of the UV radiation that Earth receives from the Sun.
Learn more about the atmosphere, here:
https://brainly.com/question/29379794
#SPJ1
Calculate the width of a vegetable garden with an area of 280 square feet if the garden is 15.5 feet long.
Answers
Which of the following is a potential exception to cell theory? Choose 1 answer: Fungal spore Asexual reproduction Mitochondria Amoebas
Answers
Answer:
Asexual reproduction
Explanation:
Cells are able to re produce themselves without other cells needed. If cells need others to make more then how would that work and plus we wouldn't have the theory of cells existing.
what is the purpose of time on analyzing fluid viscosity
Answers
Answer:
Gathering viscosity data on a material gives manufacturers the ability to predict how the material will behave in the real world.
Example:
If toothpaste does not have the correct viscosity, it can either be too difficult to pump out from the tube, or pump out too much.
explain hydrophobic and hydrophylic
Answers
Answer:
hydrophobic: tending to repel or fail to mix with water.
hydrophylic: having a tendency to mix with, dissolve in, or be wetted by water.
Hope it helps!!!
What ion has a +3 charge, 28 electrons and an atomic mass of 71?
Answers
The ion with a +3 charge, 28 electrons, and an atomic mass of 71 is the aluminum ion (\(Al^{3+}\)).
Aluminum (Al) typically has an atomic number of 13, which means it has 13 protons and 13 electrons in its neutral state. However, in the given ion, \(Al^{3+}\), the ion has lost three electrons, resulting in a +3 charge. This means that the ion now has 13 protons and only 10 electrons remaining, giving it a net positive charge of +3.
The atomic mass of aluminum is 26.98 atomic mass units (amu). The given ion has an atomic mass of 71 amu, which suggests that the ion has gained additional particles. In this case, the ion has also gained three neutrons, resulting in a higher atomic mass.
The total number of particles (protons, neutrons, and electrons) in the ion can be calculated by adding the number of protons (13) and the number of neutrons (3), which equals 16. Since the ion has a net charge of +3, it only contains 10 electrons.
In summary, the ion with a +3 charge, 28 electrons, and an atomic mass of 71 is the aluminum ion (\(Al^{3+}\)), which has 13 protons, 10 electrons, and 3 neutrons.
for such more questions on electrons
https://brainly.com/question/26084288
#SPJ8
Is pre ap chemistry hard in high school?
Answers
If you don't practice enough it's obviously going to be hard but if you practice enough it's going to be a piece of cake so don't think if it's going to be hard or not just think it's going to be worth the try at the very end
What is the percent by mass of magnesium in a 1000 g sample of ocean water (solution) that has 1.36 g of magnesium ions?
Answers
Answer:
So the percentage mass of Magnesium in ocean water is 0.13%.
Explanation:
brainliest pls
Mark is in charge of making change at his family’s garage sale on a hot summer day. He runs low on change and brings out a jar of pennies from the house. He keeps the lid off of the jar. Later in the day, he notices that the pennies are hot. Which statement best explains what happened?
a. Heat flowed from the surrounding hot air to the cooler air and transferred heat to the pennies by convection.
b. The pennies became hotter because light energy was transferred into thermal energy.
c. The air in the jar became cooler because heat flowed from the cooler air to the hot pennies by convection.
d. Heat flowed from the surrounding hot air to the cooler jar and transferred heat to the pennies by conduction.
Answers
The universe is divided into two according to thermodynamic, one is system over which we are doing experiments and other is surrounding that is surrounding the system. Therefore, the correct option is option A.
What is thermodynamics?Thermodynamics is the branch of physical science that deals with the relations between heat and other forms of energy (such as mechanical, electrical, or chemical energy). Thermo means heat and dynamics means flow of heat or energy from one place to another place.
Heat always flow from the source which is at high temperature to the sink which is at low temperature. The heat always from high temperature to low temperature source to maintain a equilibrium. There are total three laws that govern the thermodynamic. First, second and third laws of thermodynamic.
In the given experiment, heat flowed from the surrounding hot air to the cooler air and transferred heat to the pennies by convection.
Therefore, the correct option is option A.
to know more about thermodynamics, here:
https://brainly.com/question/21858980
#SPJ1
Individual Laboratory Follow-up Questions a. Explain the purpose of the experiment. b. What are reasonable sources of error for determining calcium ion concentration by the titration process? c. Summarize the conclusions for your experiment in two to three complete sentences. Include the amount of calcium in your soil.
Answers
Solution :
a). In this experiments, we can determine the hardness of the water or \($Ca^{2+}$\) ion concentration in the sample.
b). The Possible errors are :
- During the weighing of calcium standard preparation, if the balance is not traced properly, it will cause an error.
- By dilution of standard from the stock will lead a dilution error of 0.1 mL for 100 mL.
- Misreading the liter values will give an error.
c). Depending upon the calcium content present in the soil, we conclude that the fertile percent of the soil as well as the mineral capacity of the soil.
Chlorine is heated with oxygen to form dichlorine monoxide gas. Express your answer as a chemical equation. Identify all of the phases in your answer.
Answers
Answer: 4 HCl (g) + O₂ (g) → 2 Cl₂ (g) + 2 H₂O (l)
Explanation:
4 moles of hydrogen chloride (note that it is in the gaseous phase, otherwise it would be hydrochloric acid) react with 1 mole of oxygen gas to form 2 moles of chlorine gas and 2 moles of liquid water.
To conform with the law of conservation of mass, the equation must be balanced, this means that there must be the same number of each type of atom on both sides of the arrow.
Is this helpful?
The balanced chemical reaction is:
4 HCl (g) + O₂ (g) → 2 Cl₂ (g) + 2 H₂O (l)
In first place, chlorine is heated with oxygen to form dichlorine monoxide gas. Then, this can be expressed as:
HCl (g) + O₂ (g) → Cl₂ (g) + H₂O (l)
On the other side, the Law of Conservation of Matter is also called the law of conservation of mass or the Law of Lomonósov-Lavoisier. This law postulates that "The mass is not created or destroyed, only transformed."
Then, the number of atoms that are present in the reagents has to be equal to the number of atoms present in the products.
Finally, the balanced chemical reaction is:
4 HCl (g) + O₂ (g) → 2 Cl₂ (g) + 2 H₂O (l)
Learn more:
https://brainly.com/question/15400776?referrer=searchResultshttps://brainly.com/question/13911443?referrer=searchResultshttps://brainly.com/question/13979405https://brainly.com/question/24209700?referrer=searchResults