An atom of a certain element has 33 protons, 33 electrons, and a mass number of 75. How many neutrons are in this atom?
Answers
Answer:
42 neutrons
Explanation:
Mass number = number of protons + number of neutrons
number of neutrons = Mass number - number of protons
= 75 - 33
= 42
Answer:
42 neutrons
Explanation:
Related Questions
Of the following, which is a false statement regarding chemical equilibrium? Select the correct answer below: O All reactions are reversible in theory, but it is not always feasible to produce conditions that lead to any significant formation of reactants from the products. O Some reactions favor the forward direction so much over the reverse direction that they proceed until consuming nearly all of the reactants. O A reversible reaction means a reaction that is at equilibrium between reactants and products. O A reversible reaction that is in a state of equilibrium between reactants and products is running in both the forward and reverse directions at the same rate.
Answers
The correct option is C, A reversible reaction means a reaction that is at equilibrium between reactants and products is a false statement regarding chemical equilibrium.
A reversible reaction is a chemical reaction that can occur in both directions, with the products of the reaction reacting to form the original reactants. This means that the reaction can proceed in both the forward and reverse direction, depending on the conditions of the reaction.
In a reversible reaction, the reactants and products are present in a state of dynamic equilibrium, where the rates of the forward and reverse reactions are equal. This equilibrium is governed by the principle of Le Chatelier's, which states that a system at equilibrium will respond to any changes in conditions by shifting the equilibrium to counteract those changes. Understanding reversible reactions is important in fields such as chemistry and biochemistry, as they play a crucial role in many chemical and biological processes.
To learn more about Reversible reaction visit here:
brainly.com/question/16614705
#SPJ4
True/False: Energy can’t be created or destroyed: it can only be transferred from one place to another.
Answers
Answer:
true
Explanation:
Answer:
True.
Explanation:
It is true that energy cannot be made or lost, but it can move. For example, if you touch a metal pole and get a static shock, then the energy from the pole is being transferred to you. However, energy was not made or lost in the process. So, energy cannot be made or destroyed, but it can be moved. This also means there was the same amount of energy in the universe as there was millions of years ago. Hope this helps!
Sheldon from The Big Bang Theory was concerned about eating blueberries for fear of getting too many Your answer a.sugars b.lipids c. proteins d.antioxidants Submit
Answers
Sheldon's concern about eating too many blueberries is related to their (a) sugar content. Blueberries are naturally high in sugar, which can be a concern for people who are trying to limit their sugar intake.
Blueberries are also packed with antioxidants, which can help protect the body against damage from free radicals.
While Sheldon may have been worried about the sugar content of blueberries, it's important to remember that they also provide a range of important nutrients and can be a healthy addition to a balanced diet.
Sheldon's concern about blueberries may stem from his interest in nutrition and his desire to make healthy choices. While blueberries are generally considered a healthy food, they do contain a significant amount of sugar.
This can be a concern for people who are trying to manage their blood sugar levels or who are following a low-sugar diet.
However, blueberries are also rich in antioxidants, which can help protect the body against damage from free radicals. Overall, blueberries can be a healthy addition to a balanced diet, but it's important to be mindful of their sugar content and to enjoy them in moderation.
For more such questions on sugar, click on:
https://brainly.com/question/20320642
#SPJ11
The Big Bang Theory's Sheldon was worried about eating blueberries since he didn't want to consume too much is option d. antioxidants.
Sheldon's concern about eating too many blueberries was related to their high antioxidant content. Antioxidants are compounds that protect the body's cells from damage caused by free radicals, which are unstable molecules that can harm cells and contribute to the development of diseases such as cancer and heart disease. While blueberries are high in natural sugars, lipids, and proteins, Sheldon's concern was specifically related to the potential for consuming too many antioxidants, which can have negative effects on the body in large quantities.
The most popular cosmological hypothesis regarding the universe's creation is the Big Bang theory. It implies that the universe was once a singularity—a region of infinite density and temperature—and that it has subsequently expanded and cooled. The cosmic microwave background radiation is seen as proof for the Big Bang in the theory, which states that the cosmos is continually expanding and cooling now. The amount of light elements predicted by the theory is consistent with observations of the universe. The Big Bang idea is largely regarded by the scientific world as the most compelling proposal for the creation of the universe, despite the fact that many questions and mysteries remain about it.
Learn more about big bang theory here:
https://brainly.com/question/30437346
#SPJ11
_Ch7H16+_O2=_CO2+_H2O balancing the equation
Answers
Answer:
0,11,7,8
Explanation: hope this helps!
Put the steps of glucose utilization by anaerobic metabolism in order as they occur in the body.
(Bottom to Top)
1. Liver releases glucose into bloodstream or uses it to make glycogen. 2 Liver removes lactate from blood and can convert it to glucose, 3. Lactic acid accumulates in muscles and accumulated lactic acid converts to lactate, 4. Pyruvate is converted to lactic acid which produces a small amount of ATP. 5.. When oxygen is not present, muscle cells metabolize glucose to pyruvate.
Answers
The order of the steps of glucose utilization by anaerobic metabolism as they occur in the body is as follows:
1, 5, 4, 3 and 2.
Liver releases glucose into the bloodstream or uses it to make glycogen. When oxygen is not present, muscle cells metabolize glucose to pyruvate. Pyruvate is converted to lactic acid which produces a small amount of ATP. Lactic acid accumulates in muscles, and accumulated lactic acid converts to lactate. Liver removes lactate from the blood and can convert it to glucose.
When oxygen is not present in muscle cells, glucose is metabolized by anaerobic metabolism. The steps of glucose utilization by anaerobic metabolism in the body occur in the following order, from bottom to top:
Step 1: Liver releases glucose into the bloodstream or uses it to make glycogen.
Step 2: When oxygen is not present, muscle cells metabolize glucose to pyruvate.
Step 3: Pyruvate is converted to lactic acid which produces a small amount of ATP.
Step 4: Lactic acid accumulates in muscles, and accumulated lactic acid converts to lactate.
Step 5: Liver removes lactate from the blood and can convert it to glucose.
In summary, glucose utilization by anaerobic metabolism occurs when there is a lack of oxygen in the muscle cells. The liver plays a critical role in this process by releasing glucose into the bloodstream, removing lactate from the blood, and converting lactate to glucose. The conversion of pyruvate to lactic acid produces a small amount of ATP, which can provide energy for the body in the absence of oxygen. These steps enable the body to continue functioning even when oxygen is not available.
To know more about anaerobic metabolism, refer to the link below:
https://brainly.com/question/15464346#
#SPJ11
100 cm³ of a gas at 27°C is cooled to 20°C at constant pressure .Calculate the volume of gas at 20°C.
Answers
According to Charle's law, the volume of the given mass of a gas is directly proportional to its absolute temperature provided that the pressure is constant. Mathemically;
\(\begin{gathered} V\alpha T \\ V=kT \\ k=\frac{V}{T} \\ k=\frac{V_1}{T_1}=\frac{V_2}{T_2} \end{gathered}\)where;
V1 and V2 are the initial and final volume of the gas
T1 and T2 are the initial and final temperatures of the gas (in Kelvin)
Given the following parameters:
\(\begin{gathered} V_1=100\operatorname{cm}^3 \\ T_1=27^0C=27+273=300K \\ T_2=20^0C=20+273=293K \\ V_2=\text{?} \end{gathered}\)Substitute the given parameters into the formula;
\(\begin{gathered} V_2=\frac{V_1T_2}{T_1}^{} \\ V_2=\frac{100\times293}{300} \\ V_2=\frac{29300}{300} \\ V_2=\frac{293}{3} \\ V_2=97.67\operatorname{cm}^3 \end{gathered}\)Therefore the volume of the gas at 20°C is approximately 97.67cm³
What is the number of significant figures in each of the following measured quantities? 0.0105 L.
Answers
The measured quantity 0.0105 L has three significant figures. Significant figures are the digits in a measurement that convey precision, excluding leading zeros and trailing zeros without a decimal point.
In the measured quantity 0.0105 L, there are three significant figures. Significant figures are the digits in a measurement that indicate the precision and reliability of the value. The general rule for determining significant figures is as follows:
1. Non-zero digits are always significant. In this case, the digits "1", "0", and "5" are all non-zero and therefore significant.
2. Leading zeros (zeros at the beginning of a number) are not significant; they act as placeholders. In this measurement, the leading zero before the decimal point is not considered significant.
3. Zeros between significant digits are significant. There are no zeros between the significant digits "1", "0", and "5" in this case.
4. Trailing zeros (zeros at the end of a number) after a decimal point are significant. In this measurement, the trailing zero after the "5" is significant.
By applying these rules, we can determine that the measured quantity of 0.0105 L has three significant figures, representing the precision of the measurement to the hundredth place.
learn more about measured quantity here:
https://brainly.com/question/29135463
#SPJ11
what are examples of soluble salts (metal+acids)
Answers
Answer:
Chloride salts - made of HCI
Nitrate salts - made of HNO3
Sulfate salts - made of H2SO4
These are all the examples I could think of.
1. if 25.0 grams of barium nitrate are placed in 100.0 ml of 2.00 m sodium sulfate, a. how many grams of precipitate will theoretically form?
Answers
If 25.0 grams of barium nitrate are placed in 100.0 ml of 2.00 M sodium sulfate, The grams of precipitate will theoretically form is 20.97 g.
The reaction is given as :
Ba(NO₃)₂ + Na₂SO₄ -----> BaSO₄
given that :
mass of Ba(NO₃)₂ = 25 g
molarity of Na₂SO₄ = 2 M
volume = 100 mL = 0.1 L
moles of Na₂SO₄ = molarity × volume
= 2 × 0.1
= 0.2 mol
moles of Ba(NO₃)₂ = mass / molar mass
= 25 / 261.33
= 0.09 mol
Ba(NO₃)₂ is limiting reagent. therefore , the 1 mole of Ba(NO₃)₂ produce 1 mole of BaSO₄.
moles of BaSO₄ = 0.09 mol
mass of BaSO₄ = moles × molar mass
= 0.09 × 233
= 20.97 g
The theoretically yield = 20.97 g
Thus, If 25.0 grams of barium nitrate are placed in 100.0 ml of 2.00 M sodium sulfate, The grams of precipitate will theoretically form is 20.97 g.
To learn more theoretically yield here
https://brainly.com/question/14966377
#SPJ4
What are the 4 steps of the enzymatic cycle?
Answers
The enzymatic cycle has four steps, and they include the following;
1. The reaction between the Enzyme and Substrate
2. The substrate/enzyme complex formation
3. Catalysis
4. Enzyme releases a product
A small molecule will attach to the enzyme's active site and stop the action. The plants adapt by changing amino acid(s) in the enzyme. They adjust the structure and are continuously active; the small molecule cannot limit this enzyme.
The four steps in an enzyme cycle are;
1. The substrate and enzyme are found in one region. There are times when there is more than one substrate molecule and the enzyme changes.
2. The enzyme will then be trapped on the substrate in the special region called the active site. The combination is called substrate/enzyme complex. The active site will be in a shaped special region for the enzyme, which fits around a substrate.
3. Catalysis will happen when the Substrate changes. It can be broken down or combined with other molecules forming something new. It will break and form chemical bonds; afterward, a product/enzyme complex will occur.
4. The enzyme will release a product. When the enzyme is relaxed, it will return to its original shape and be ready to work on the other substrate molecule.
To learn more about the enzyme, refer;
brainly.com/question/1596855
#SPJ4
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
Which of the following must be
TRUE if a solution is to be
considered acidic?
A. [H^+] < [OH)
B. [H^+] > [OH ]
C. KW= [H^+] /[OH]
D. [H^+] =[OH)
Answers
Answer:
c
Explanation:
kw=h+bls
the molar enthalpy of vaporization of hexane (c6h14) is 28.9 kj/mol, and its normal boiling point is 68.73 °c. what is the vapor pressure of hexane at 25.00 °c?
Answers
The vapor pressure of hexane at 25.00 °C is approximately 0.292 atm.
To calculate the vapor pressure of hexane at 25.00 °C, we can use the Clausius-Clapeyron equation:
\(ln(P2/P1) = (-ΔHvap/R) * (1/T2 - 1/T1)\)
Where:
P1 is the vapor pressure at the boiling point (68.73 °C) (unknown)
P2 is the vapor pressure at the desired temperature (25.00 °C)
ΔHvap is the molar enthalpy of vaporization (28.9 kJ/mol)
R is the ideal gas constant (8.314 J/(mol·K))
T1 is the boiling point temperature in Kelvin (68.73 + 273.15)
T2 is the desired temperature in Kelvin (25.00 + 273.15)
Rearranging the equation, we get:
\(P2/P1 = e^((-ΔHvap/R) * (1/T2 - 1/T1))\)
To find P1, we can rearrange the equation further:
\(P1 = P2 / e^((-ΔHvap/R) * (1/T2 - 1/T1))\)
Substituting the given values into the equation:
\(P1 = P2 / e^((-28.9 kJ/mol / (8.314 J/(mol·K))) * (1/(25.00 + 273.15) - 1/(68.73 + 273.15)))\)
Calculating the right-hand side of the equation and substituting P2 = 1 atm (since it's the standard pressure):
\(P1 = 1 atm / e^((-28.9 kJ/mol / (8.314 J/(mol·K))) * (1/(25.00 + 273.15) - 1/(68.73 + 273.15)))\)
P1 ≈ 0.292 atm
Learn more about vapor pressure here :-
https://brainly.com/question/29640321
#SPJ11
CH3CH2C=CH2)
O=O
?
What is the functional group for this compound
Answers
Answer:
ketone
Explanation:
please mark me as brainlist
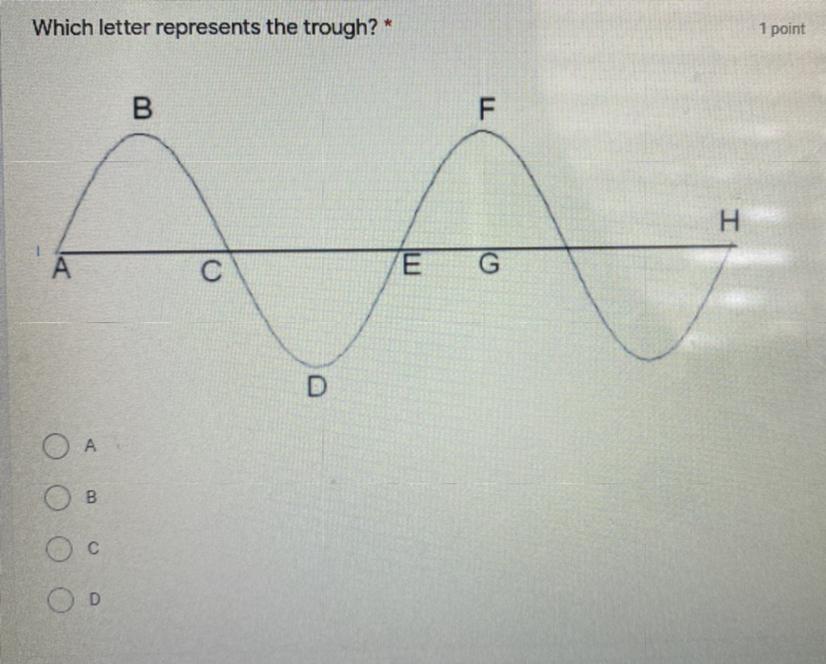
What letter represents the trough? :) PLEASE FAST!
1) A
2) B
3) C
4) D
Answers
If the concentration of reactants is decreased, how is the reaction rate affected?A.) the reaction rate decreasedB.) the reaction rate can increase or decreaseC.) the reaction rate does not changeD.) the reaction rate increases
Answers
Answer:
A.) The reaction rate decreased.
Explanation:
Let's review the rate law based on the effect on concentration on reactions: typically, reaction rates decrease with time because reactant concentrations decrease as reactants are converted to products. Reaction rates generally increase when reactant concentrations are increased.
Based on this logic, the reaction rate when the concentration of reactants is decreased, this would have a decreased too. The answer would be A.) The reaction rate decreased.
standard units of measure for density
Answers
Answer:
g/cm³ for solids,
g/ml for liquids
g/L for gases.
Explanation:
Though SI unit of density is kg/m³, for convenience we use g/cm³ for solids, g/ml for liquids and g/L for gases. Mathematically, density is defined as mass divided by volume: ρ=m/V
a sample of a molecular compound was analyzed and found to contain 0.707 grams carbon (c), 0.2372 grams of hydrogen (h). determine the empirical formula of the compound. given the added information that the molar mass of the compound is 8 times the empirical mass, determine the molar mass of the compound.
Answers
The molar mass of the compound is 128.4 g/mol.
The empirical formula is an empirical formula that represents the lowest whole-number ratio of the atoms present in a compound. The empirical formula for the molecular compound is calculated using the percentage composition of the elements present in the compound. The steps used to find the empirical formula are as follows:
Find the mass of each element present in the compound.Convert each mass to moles.Divide each mole value by the smallest number of moles.Round to the nearest whole number and write the subscripts.The molar mass is the mass of a substance that contains 6.02 × 10²³ atoms or molecules. To calculate the molar mass of a compound, add the masses of all the atoms present in the compound.
C=0.707g,12.01 g/mol=0.0588 molCnH=0.2372 =1.01g/m=0.235 mol H
nH=4nC
The empirical formula of the compound is CH4. The molar mass of the compound can be calculated using the empirical formula.
M=12.01 g/mol+4(1.01 g/mol)=16.05 g/mol
The molar mass of the compound is 8 times the empirical mass, so the actual molar mass is;
M=8(16.05{g/mol})=128.4g/mol. The molar mass of the compound is 128.4 g/mol.
More on molar mass: https://brainly.com/question/15583536
#SPJ11
if five elements have atomic numbers 2,3,6,9 which two numbers are the most reactive??
Answers
Explanation:
\(element \: with \: 2 \: atomic \: number \\ \\ is \: stable \: because \: it \: has \: already \: \\ \\ completed \: its \: orbit \: \\ \\ but \: atomic \: number \: with \: 3 \: and \: 9 \\ \\ will \: have \: to \: become \: stable \: \\ \\ after \: giving \: one \: electron \\ \\ so \: \: element \: with \: 3 \: and \: 9 \: atomic \: number \\ \\ is \: most \: reactive\)
When fluorine gas is put into contact with calcium metal at high temperatures, calcium fluoride powder is created in an exothermic reaction
Answers
Answer:
True,When fluorine gas is put into contact with calcium metal at high temperatures, calcium fluoride powder is created in an exothermic reaction.
How many moles are in 2.5 * 10^20 iron atoms?
Answers
Answer:
The answer is 0.0004 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\ \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{2.5 \times {10}^{20} }{6.02 \times {10}^{23} } \\ = 0.000415282...\)
We have the final answer as
0.0004 molesHope this helps you
what three types of evdince did mosa mack find to supporut the thoery of evolition
Answers
I recommend you reword this because of plagiarism. :)
Please Help I need to finish this by the end of the day!

Answers
Answer:
C
Explanation:
If I am not mistaken, due to the conservation of mass, whatever mass is on the side of the reactants should be the same for the product side.
This means
218.76g+53.96g = 272.72g
272.72g-266.66g = 6.06g
So the answer should be C
4. One of Dr. Birdley's boxes has a volume
of 240 cm^3 and a density of 15 g/cm^3.
The mass of the box is
a) 225 g
b) 265 g
c)3600 g
d) 32 g
Answers
Density = Mass/Volume but it can also be rearranged to:
Mass = Volume x Density
Given in the question:
Volume - 240 cm3
Density - 15 g/cm3
Calculation
Mass = Volume x Density
= 240 x 15
= 3600g
Therefore, the mass of the box is 3600g so the answer is C.
what is pyruvate converted to
Answers
Answer:
gold
Explanation:
The graphic represents the Milky Way galaxy.
Which label best represents the location of our solar system within the milky way galaxy?
A
B
C
D
Answers
Answer:
planets,meteoroids and asteroids
Explanation:
For biomass, solar, coal, natural gas, oil, and geothermal energy, identify each energy resource as renewable or non-renewable.
Answers
Answer:
There are nine major kinds of natural resources found on the planet Earth, and all these nine major resources come under two categories, that is, renewable and nonrenewable. The renewable resources refer to the resources that get regenerated again and again over a short time duration. These include five major resources, that is, wind, solar, hydro, geothermal, and biomass.
On the other hand, nonrenewable energy resources are the ones that are present in a very limited amount, as it takes a very long time to regenerate again. The general kinds of nonrenewable energy resources are nuclear, coal, oil, and natural gas.
Hence, biomass, solar, and geothermal energy comes under the renewable resources category, and coal, natural gas, and oil come under renewable resources category.
Answer: the verified answer from an expert.
Explanation:
If 60 g of a radioactive substance naturally decays to 15 g after 16 hours, what is the half-life of the radioisotope?.
Answers
The half-life of the radioisotope is 8 hours.
What do you mean by half-life?
Half-life, in radioactivity, is the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay (change spontaneously into other nuclear species by emitting particles and energy), or, equivalently, the time interval required for the number of disintegrations per second of a radioactive.
How do you calculate half-life decay?
The time required for half of the original population of radioactive atoms to decay is called the half-life. The relationship between the half-life, T1/2, and the decay constant is given by T1/2 = 0.693/λ.
The half-life of the radioisotope is 8 hours.
Half life=total life/2
Half life=16/2
Half life=8 hours
Thus, the half-life of the given radioisotope is 8 hours.
To know more about half-life:
https://brainly.com/question/19337635
#SPJ4
(a) identify the individual who most likely exhibits symptoms of cystinuria. 0 / 10000 word limit question 2 (b) describe the relationship between the total number of mutant alleles in an individual and the concentration of cysteine in the urine. 0 / 10000 word limit question 3 (c) evaluate the hypothesis that mutations in slc7a9 have a greater effect on the transport of cysteine across the plasma membrane of kidney cells than do mutations in slc3a1 . 0 / 10000 word limit question 4 (d) explain how the data support the claim that cysteine is a large polar molecule.
Answers
(a) Cystinuria is an inherited disorder that affects the transport of cysteine, an amino acid, in the kidneys.
(b) Individuals with two mutant alleles for the genes involved in cysteine transport (homozygous) will have a higher concentration of cysteine in their urine compared to individuals with only one mutant allele (heterozygous).
(c) Both slc7a9 and slc3a1 genes code for proteins involved in the transport of cysteine in the kidneys.
(d) The reactivity and the presence of polar functional groups contribute to the overall polarity of cysteine.
(a) Cystinuria is an inherited disorder that affects the transport of cysteine, an amino acid, in the kidneys. Individuals with cystinuria can experience symptoms such as recurrent kidney stones. The person most likely to exhibit symptoms of cystinuria would be someone who has inherited two mutant alleles for the genes involved in cysteine transport.
(b) The relationship between the total number of mutant alleles in an individual and the concentration of cysteine in the urine is as follows: Individuals with two mutant alleles for the genes involved in cysteine transport (homozygous) will have a higher concentration of cysteine in their urine compared to individuals with only one mutant allele (heterozygous). This is because the mutated transporters are less effective in reabsorbing cysteine from the urine, leading to higher levels of cysteine excretion.
(c) The hypothesis that mutations in slc7a9 have a greater effect on the transport of cysteine across the plasma membrane of kidney cells than mutations in slc3a1 can be evaluated by studying the function of these genes and their respective mutations. Both slc7a9 and slc3a1 genes code for proteins involved in the transport of cysteine in the kidneys.
(d) The claim that cysteine is a large polar molecule can be supported by data that demonstrates its chemical properties. Cysteine contains a thiol group (-SH) in its structure, which is a polar functional group. The thiol group is highly reactive and prone to forming disulfide bonds with other cysteine molecules. This reactivity and the presence of polar functional groups contribute to the overall polarity of cysteine.
Experimental evidence, such as X-ray crystallography or spectroscopic analysis, can provide insights into the molecular structure and properties of cysteine, confirming its size and polarity. Additionally, data on the solubility and interactions of cysteine in different solvents can also support the claim of cysteine being a large polar molecule.
Learn more about cysteine from the link given below.
https://brainly.com/question/31839509
#SPJ4
select whihc of the following standard entahlpy of formation values is not zer oat 25 mn ch4 fe cl2 n2
Answers
The standard enthalpy of formation for CH4, Fe, and Cl2 are all zero. The standard enthalpy of formation for N2 is not zero, as it is equal to -57.6 kJ/mol.
What is enthalpy?
A thermodynamic system's enthalpy, which is one of its properties, is calculated by adding the system's functional energy to the product of the its pressure and volume. It is a state function that is frequently used in measurements of chemical, biological, as well as physical systems at constant pressure, which the sizable surrounding atmosphere conveniently provides. The pressure-volume term describes the effort needed to create space for the system by displacing it's own surroundings in order to determine its physical dimensions. For solids and liquids under typical conditions, the pressure-volume word is very small, whereas it is only moderately small for gases. Therefore, enthalpy serves as a stand-in for energy throughout chemical systems; enthalpy differences are what are commonly referred to as bond, lattice, and solvation "energies" in chemistry.
To learn more about enthalpy
https://brainly.com/question/14291557
#SPJ4