an aqueous solution at 25 has a poh of 4.5. calculate the ph. round your answer to 1 decimal places.
Answers
The pH of an aqueous solution can be calculated using the pH scale, which is a logarithmic scale that ranges from 0 to 14.
A solution with a pH of 7 is considered neutral, while solutions with pH values less than 7 are considered acidic and solutions with pH values greater than 7 are considered basic. The pH is calculated using the formula pH = 14 - pOH, where pOH is the negative logarithm of the hydroxide ion concentration [OH-]. Given that an aqueous solution at 25 has a pOH of 4.5, we can calculate the pH as follows: pOH = 4.5[OH-] = 10^-4.5[OH-] = 3.16 x 10^-5 (since 10^-4.5 = 3.16 x 10^-5).
Using the formula pH = 14 - pOH, we can substitute in the value for pOH and calculate the pH: pH = 14 - 4.5pH = 9.5. Therefore, the pH of the aqueous solution is 9.5 when the pOH is 4.5. The pH of the solution can also be considered basic because its pH value is greater than 7.
To know more about aqueous solution refer to:
https://brainly.com/question/19587902
SPJ11
Related Questions
the molecule that provides the energy to drive endergonic reactions in the body is abbreviated
Answers
The molecule that provides the energy to drive endergonic reactions in the body is abbreviated as ATP, which stands for adenosine triphosphate. ATP is a high-energy molecule that serves as the primary source of energy for various cellular processes and reactions.
Endergonic reactions are those that require an input of energy to proceed. In biological systems, this energy is often provided by ATP. The ATP molecule is composed of a nitrogenous base (adenine), a sugar (ribose), and three phosphate groups. The energy stored in ATP is mainly found in the bonds between the phosphate groups.
When a cell needs energy for an endergonic reaction, ATP undergoes hydrolysis, a process in which a phosphate group is removed from the molecule, resulting in the formation of adenosine diphosphate (ADP) and an inorganic phosphate (Pi). This reaction releases the energy that can be utilized to power various cellular processes, such as muscle contraction, protein synthesis, and cellular transport.
Conversely, the energy released during exergonic reactions (reactions that release energy) can be harnessed to regenerate ATP from ADP and Pi. This continuous cycle of ATP hydrolysis and regeneration ensures that cells have a constant supply of energy to drive endergonic reactions and maintain various biological functions.
In summary, ATP is the key molecule that provides the energy required for endergonic reactions in the body. It acts as a universal energy currency, allowing cells to store, transfer, and utilize energy efficiently for a wide range of cellular processes.
learn more about adenosine triphosphate here: brainly.com/question/28431482
#SPJ11
N204(0) + 2NO2(g)
Colorless Brown
Keq = 6.16 x 103
What is the predicted direction of change?
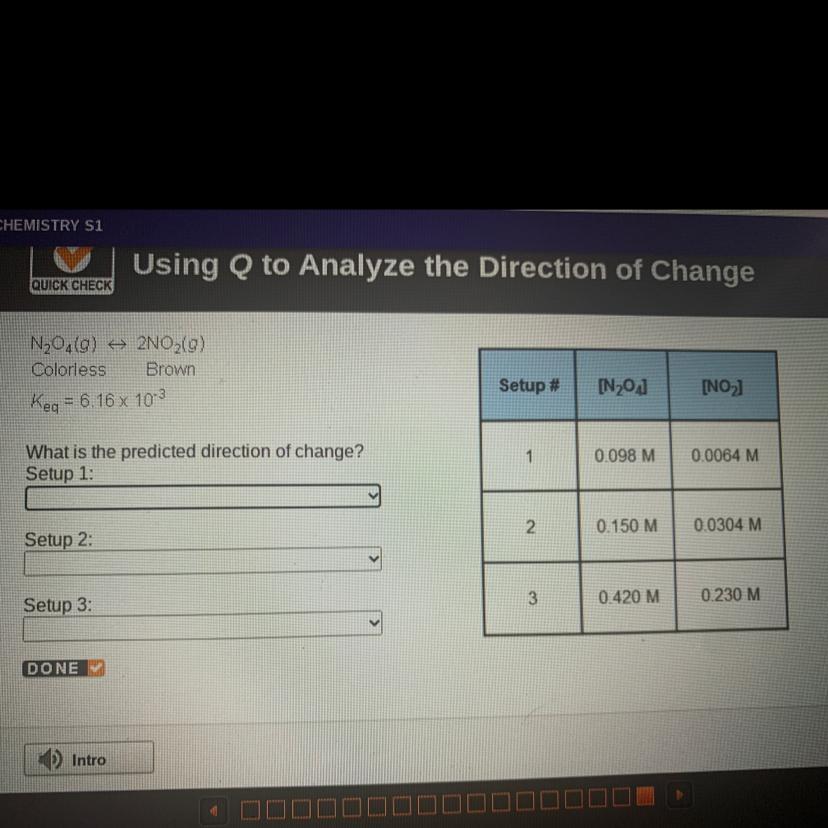
Answers
setup 1 : to the right
setup 2 : equilibrium
setup 3 : to the left
Further explanationThe reaction quotient (Q) : determine a reaction has reached equilibrium
For reaction :
aA+bB⇔cC+dD
\(\tt Q=\dfrac{C]^c[D]^d}{[A]^a[B]^b}\)
Comparing Q with K( the equilibrium constant) :
K is the product of ions in an equilibrium saturated state
Q is the product of the ion ions from the reacting substance
Q <K = solution has not occurred precipitation, the ratio of the products to reactants is less than the ratio at equilibrium. The reaction moved to the right (products)
Q = Ksp = saturated solution, exactly the precipitate will occur, the system at equilibrium
Q> K = sediment solution, the ratio of the products to reactants is greater than the ratio at equilibrium. The reaction moved to the left (reactants)
Keq = 6.16 x 10⁻³
Q for reaction N₂O₄(0) ⇒ 2NO₂(g)
\(\tt Q=\dfrac{[NO_2]^2}{[N_2O_4]}\)
Setup 1 :
\(\tt Q=\dfrac{0.0064^2}{0.098}=0.000418=4.18\times 10^{-4}\)
Q<K⇒The reaction moved to the right (products)
Setup 2 :
\(\tt Q=\dfrac{0.0304^2}{0.15}=0.00616=6.16\times 10^{-3}\)
Q=K⇒the system at equilibrium
Setup 3 :
\(\tt Q=\dfrac{0.230^2}{0.420}=0.126\)
Q>K⇒The reaction moved to the left (reactants)
Answer:
The system will shift toward the products
The system is at equilibrium
The system will shift toward the reactants
Explanation:
This is correct on edg... Good Luck!!!!
How many moles of HCl can be produced from 0.207 g of SOC12 SOC12(1) + H2O(1) = SO2(g)+ 2HCl(g)
Answers
Answer:
.80*10⁻³ moles of HCl can be produced from 0.226 g of SOCl₂
Explanation:
The balanced reaction is:
SOCl₂ + H₂O ----> SO₂ + 2 HCl
By stoichiometry of the reaction they react and produce:
SOCl₂: 1 mole
H₂O: 1 mole
SO₂: 1 mole
HCl: 2 mole
Being:
S: 32 g/mole
O: 16 g/mole
Cl: 35.45 g/mole
H: 1 g/mole
the molar mass of the compounds participating in the reaction is:
SOCl₂: 32 g/mole + 16 g/mole + 2*35.45 g/mole= 118.9 g/mole
H₂O: 2*1 g/mole + 16 g/mole= 18 g/mole
SO₂: 32 g/mole + 2*16 g/mole= 64 g/mole
HCl: 1 g/mole + 35.45 g/mole= 36.45 g/mole
Then, by stoichiometry of the reaction, the following amounts of mass react and are produced:
SOCl₂: 1 mole* 118.9 g/mole= 118.9 g
H₂O: 1 mole* 18 g/mole= 18 g
SO₂: 1 mole* 64 g/mole= 64 g
HCl: 2 mole* 36.45 g/mole= 72.9 g
Then the following rule of three can be applied: if by stoichiometry of the reaction 118.9 grams of SOCl₂ produce 2 moles of HCl, 0.226 grams of SOCl₂ how many moles of HCl do they produce?
moles of HCl= 3.80*10⁻³
3.80*10⁻³ moles of HCl can be produced from 0.226 g of SOCl₂
State the law of multiple proportions.
Answers
Answer:
Law of Multiple proportion states that when two elements combine to form more than one compound, the mass of one element, which combines with a fixed mass of the other element, will always be ratios of whole numbers. For example, let us assume 2 molecules CO (carbon monoxide) and CO2(carbon dioxide).
Explanation:
A chemistry student produces 1.45 grams of silver during an experiment by reacting
2.45 grams of silver nitrate with excess copper wire. The species of copper that is formed from this single replacement reaction is copper (Il) nitrate.
A. Calculate the students theoretical yield for this experiment.
B. Determine the percent yield for the student's experiment.
C. How would you rate the student's results, most excellent or poor?
D. Identify the reactant that would be left over upon the reaction reaching completion.

Answers
A single substitution reaction a strip or zinc metal is added to a copper(II) nitrate solution. Copper will be pushed out of the solution by zinc. As a result, zinc nitrate, Zn(NO3)2, and copper metal, Cu, produced.
What is a substitution reaction reaction example?An illustration of a single substitution reaction is the reaction of potassium (K) and water (H2O). In the process, hydrogen gas (H2) is released and potassium hydroxide (KOH), a colourless solid chemical, formed. 2K + 2H2O 2KOH + H is the reaction's equation.
What other names are given to copper II nitrate?This page explains the Copper II Nitrate formula, sometimes referred to as Cupric nitrate or Copper Dinitrate formula. This inorganic substance exists as a solid with blue crystals. Cu(NO3)2 is the chemical formula for Copper II Nitrate.
To know more about zinc metal visit:
https://brainly.com/question/13683969
#SPJ1
What type of narrow, curved rock formation forms when a sea cave is continually eroded?
Answers
Sea Arches are formed when a cave continues to be eroded and expanded until it cuts through a headland.
What is produced when a sea cave is eroded continually?A sea cave forms when a sea arch pursues to be eroded and widened until the rock becomes too weak to carry the roof of the sea cave and collapses into the sea. Caves are devised by the dissolution of limestone. Rainwater collects carbon dioxide from the air and as it percolates through the soil, which rotates into a weak acid.
Sea caves occur on almost every precipice headland or coast where the waves break straight on a rock cliff and are formed Sea caves by mechanical erosion rather than the chemical solution procedure that is responsible for the majority of inland caves.
So we can conclude that Sea caves or littoral caves are formed fundamentally from erosion caused by waves
Learn more about Sea caves here: https://brainly.com/question/11986692
#SPJ1
Do different color lights (RBG) use additive or subtractive properties to bring light of a certain color to our eyes?
Answers
Answer:
The lighting filters use subtractive properties to bring light of a color to the eye because subtractive color mixing uses white light source with a series of filter to eliminate certain wavelengths of light.
Different colour lights (RBG) uses additive properties to bring light of a certain color to our eyes.
What is color synthesis?Color synthesis is the art of forming different types of colours from small number of primary colours. The color formed is called a secondary color.
Color synthesis is carried out through the following means:
Additive color synthesis andSubtractive color synthesis.In additive color synthesis, the three primary colours lights that are combined is Red(R), Blue (B) and Green(G).
The wavelength from the various color light combine to generate white light which is visible to the eye.
Therefore, different colour lights (Red, Blue and Green) uses additive properties to bring light of a certain color(white) to our eyes.
Learn more about color synthesis here:
https://brainly.com/question/18452279
1. The respiratory system depends on the nervous system for signals to:
Answers
Answer:transfer oxygen through your blood
Explanation:
Answer:
coordinate the muscles that control breathing
hope this helps ⭐
PLEASE HELP URGENT!!
What is the SI system? What are its benefits?
Answers
Answer:
The SI system : The international system of units.
It is a scientific method of expressing the magnitudes or quantities of important natural phenomena.
Benefits of SI system
It is a coherent system of units ( a system based on certain sets of fundamental units )It is a rational system of units ( it assigns only one unit to a particular physical quantity )




Why open flames near to ethanol can lead to serious fires?
Answers
\(\huge\bold\red{Aɴswᴇʀ}\)
Ethanol is a polar solvent, a flammable liquid made of gasoline and alcohol that fully and immediately combines with water instead of separating, which is the opposite of what a firefighter attempting to knock down a car fire might expect.
Answer:
Ethanol has a high vapor pressure. Therefore, even though you may not be able to see the particles of ethanol in the air, they are there and will readily fuel a combustion reaction if an open flame is nearby
Extra points please someone help

Answers
Answer: Plants and animals share many characteristics, but they are different in some respects hope that helps-
Explanation:
The visible lines from hydrogen are all members of the: a. Lyman series. b. Balmer series. c. Paschen series. d. Brackett series. e. Pfund series.
Answers
The visible lines from hydrogen are all members of the Balmer series. That is option B.
What are visible lines of hydrogen?The hydrogen is an atom that is capable of producing visible spectral lines that corresponds to transitions from higher energy levels through it.
The Balmer series is the part of the lines emitted from a hydrogen atom that contains four lines of visible spectrum called Balmer series.
The four lines possess that following wavelengths such as 410 nm, 434 nm, 486 nm and 656 nm.
Learn more about wavelength here:
https://brainly.com/question/29213586
#SPJ1
Solid lead nitrate is heated and forms solid lead oxide, gaseous nitrogen dioxide, and oxygen.
Answers
Solid lead nitrate is heated and forms solid lead oxide, gaseous nitrogen dioxide, and oxygen then it form of decomposition reaction
Lead nitrate is a white color inorganic powder with the chemical formula Pb ( NO₃)₂ when Lead nitrate decomposes it produces Lead oxide a yellow colored oxide of brown colored Nitrogen dioxide gas, and colorless Oxygen gas and also it gives yellow colors
When lead nitrate is heated it decomposes to form lead oxide, nitrogen dioxide and oxygen from the reaction, we can see that lead nitrate decomposes on heating and forms lead oxide, nitrogen dioxide and oxygen thus, lead nitrate on decomposition gives lead oxide, nitrogen dioxide and oxygen and the lead nitrate and when solid lead nitrate heated it decomposes to produce light yellow solid lead monoxide, reddish-brown nitrogen dioxide gas and colourless oxygen gas
Know more about lead nitrate
https://brainly.com/question/4588222
#SPJ1
the firm outer covering of a plant cell is a
A.cell body
B.cell membrane
C.cell wall
d.cell structure
Answers
Answer:
it is the cell wall :)
Explanation:
Answer: The answer is C
) Consider the general reaction 5Br−(aq)+BrO3−(aq)+6H+(aq)→3Br2(aq)+3H2O(aq) For this reaction, the rate when expressed as Δ[Br2]/Δt is the same as A) −5Δ[Br−]/Δt B) −0.6Δ[Br−]/Δt C) 3Δ[BrO3−]/Δt D) −Δ[H2O]/Δt E) None of these choices are correct.
Answers
The correct choice is A) −5Δ[Br−]/Δt. The rate expressed as Δ[Br2]/Δt is proportional to -5 times the rate of change of Br−.
In the given reaction 5Br−(aq) + BrO3−(aq) + 6H+(aq) → 3Br2(aq) + 3H2O(aq), the stoichiometric coefficients provide information about the relationship between the reactants and products. To determine the rate expressed as Δ[Br2]/Δt, we need to compare it with the rate of change of the other species.
Based on the balanced equation, for every 5 moles of Br− consumed, 3 moles of Br2 are produced. Therefore, the rate of change of Br−, Δ[Br−]/Δt, is related to the rate of change of Br2, Δ[Br2]/Δt, by a factor of -5/3.
The other choices, B) −0.6Δ[Br−]/Δt, C) 3Δ[BrO3−]/Δt, and D) −Δ[H2O]/Δt, do not correspond to the correct relationship based on the stoichiometric coefficients of the reaction. Therefore, the correct answer is A) −5Δ[Br−]/Δt.
for such more questions on reaction
https://brainly.com/question/24795637
#SPJ8
anhydrous sodium sulfate is used at the end of the procedure to dry the product. what are the intermolecular forces at play that allow the sodium sulfate to dry the product?
Answers
The intermolecular forces at play that allow the sodium sulfate to dry the product are hydrogen bonding and van der Waals forces.
When anhydrous sodium sulfate is added to a solution, it readily absorbs water molecules by forming hydrogen bonds with them. This removal of water molecules results in a reduction in the number of water molecules present in the mixture and thus, the product is dried.
Additionally, van der Waals forces also play a role in the drying process. Van der Waals forces are weak, non-covalent forces that arise due to the fluctuation of electron density in a molecule. They are responsible for the interaction between molecules, including the attraction between sodium sulfate and water molecules.
The combined effect of hydrogen bonding and van der Waals forces results in the absorption of water by the anhydrous sodium sulfate, leading to the drying of the product.
Learn more about intermolecular forces here: https://brainly.com/question/13588164
#SPJ4
Hydrogen and oxygen react chemically to form water. How much water would form if 4. 8 grams of hydrogen reacted with 38. 4 grams of oxygen?.
Answers
Quantity of water form will be 36 gram
We need a stoichiometric equation for water synthesis:
H2(g) + 1/2O2(g) → H2O(l)
Clearly, dihydrogen must be present in a 2:1 molar ratio with respect to dioxygen.
Moles of dihydrogen = 4.8/2 = 2.4 moles
Moles of dioxygen = 38.4/32 = 1.2 moles
Given these molar quantities (they are approx. 2:1) the gases are present in stoichiometric proportion. 2mol water are going to be produced.
2 mol x 18g/mol = 36 gram
So, quantity of water form will be 36 gram
Learn more about Water reaction here:
https://brainly.com/question/25597694
#SPJ4
11. How is the atomic emission spectrum of an element produced?
Answers
Answer:
Atomic emission spectra are produced when excited electrons return to the ground state. When electrons return to a lower energy level, they emit energy in the form of light.
Explanation:
Which area of science is considered the central science?
chemistry
biology
physics
environmental science
Answers
Answer:
Chemistry
Explanation:
Chemistry is considered the central science.
Which area of science is considered the central science?Chemistry is sometimes called "the central science" because it's so important to other fields of science, like biology, geology, astronomy, physics, medicine, engineering, materials science, and many other areas of study.
Which branch of science is the most important?All the branches of science possess their own level of importance, However, physics deals with the fundamental workings of material reality which are central to all other branches of science.
Learn more about chemistry here https://brainly.com/question/24419453
#SPJ2
how would your calculations of the concentration of [fescn]2 been affected if the cuvette you used had a 1.5 cm path length rather than the 1.0 cm value you were told to use?
Answers
The increased distance across the cell will result in an increase absorbance reading.
The concentration of \([Fescn]_2\) would be affected if the cuvette had a 1.5 cm path length rather than the 1.0 cm value used.Since the absorbance of a sample is proportional to the concentration of a sample (as described by the Beer-Lambert law), increasing the path length of the cuvette would result in a decrease in absorbance. This means that the concentration of the sample would be lower than if the 1 cm path length was used. In other words, the concentration of \([Fescn]_2\)would be lower if the cuvette had a 1.5 cm path length than if it had a 1.0 cm path length.
learn more about cuvette Refer:brainly.com/question/29385690
#SPJ1
Carbon dioxide (CO2) is a common compound made of
A)3 elements and a total of 3 atoms
B)2 elements and a total of 2 atoms
C)3 elements and a total of 2 atoms
D)2 elements and a total of 3 atoms
Answers
To make a type of kidney stone, calcium oxalate, you are given 1 mole of CaCl2 and 1 mole Na2C,04 as reactants. Predict the products and balance the chemical reaction. What are the products and their mole coefficients in the balanced chemical equation? a. 2 mole of Ca(C2O4)2(s) and 1 mole of NaCl (aq) b. 1 mole of CaC204(s) and 2 mole of NaCl(aq) c. 1 mole of Ca(C204)2(s) and 1 mole of NaCl (aq) d. 1 mole of CaC2O4(s) and 1 mole of NaCl(aq) e. 2 mole of CaC204(s) and 1 mole of NaCl(aq)
Answers
To make type of kidney stone, calcium oxalate, 1 mole of CaCl₂ and 1 mole Na₂C₂O₄ as reactants. The products and balance the chemical reaction is one mole of CaC₂O₄ and two moles of NaCl. The correct option is b.
The Calcium and the oxalate stones make up the majority of the kidney stones. Many of the people who will develop the calcium-containing stones have the excessive calcium in their urine, that is known as the hypercalciuria.
The 1 mole of CaCl₂ and 1 mole Na₂C₂O₄ reaction is as follows :
CaCl₂ + Na₂C₂O₄ ----> CaC₂O₄ + 2NaCl
This is the balanced chemical equation. Therefore the option b is correct.
To learn more about kidney stone here
https://brainly.com/question/26697997
#SPJ4
What is an Atom? please help
Answers
Answer:
An atom is the basic building block of matter. Anything that has a mass-- in other words, anything that occupies space--is composed of atoms.
Answer:
An atom is a particle of matter that uniquely defines achemical element.
Explanation:
this might help you
Which of the following are things that may change during an experiment?
Answers
Answer:
The things that are changing in an experiment are called variables. A variable is any factor, trait, or condition that can exist in differing amounts or types.
Put hydrogen bonds, dispersion forces and dipole-dipole forces in order of how strong they are and give an example of each
type of attraction.
Answers
The order of strongest to weakest is as follows hydrogen bonds, dipole-dipole forces and dispersion forces. The strongest intermolecular forces are hydrogen bonds.
The high boiling point of water is a result of this connection. These bonds are crucial to the structure of both synthetic and organic polymers. Water, or H2O, is an illustration of a hydrogen bond.
Due to the persistent polarity of molecules that exists between polar molecules at the tail and head of the molecule itself, dipole-dipole forces are rather weak. Hydrochloric acid, also known as HCL, is an illustration of dipole-dipole forces.
The momentary generated dipole that causes an unequal electron density makes dispersion forces the weakest intermolecular interactions. Methane or molecules containing CH4 are an illustration of dispersion force.
To know more about hydrogen bonds visit:
brainly.com/question/15099999
#SPJ4
Which factor least influences the rate of photosynthesis
Answers
Answer:
light intensity
Explanation:
Copper oxide (CuO) i reacted with ulfuric acid (H2SO) to make copper ulfate (CuSO4)
and water (H20). A. Calculate the total relative formula ma (Mr) for the reactant of thi reaction
Answers
The total relative formula ma (Mr) for CuO is 79 and H2SO4 is 98
What is relative formula mass?
The total of the relative atomic masses of the atoms represented by the numbers in the formula represents the relative formula mass of a substance composed of molecules.
Copper II sulfate is a cyan-blue chemical that is produced when copper (II) oxide, a black solid, reacts with sulfuric acid. Water and copper (II) sulfate are produced by the reaction of copper (II) oxide with sulfuric acid. This reaction can be categorized as either a neutralization reaction or a double displacement reaction.
CuO(s) + H2SO4 (aq) → CuSO4 (aq) + H2O(l)
Relative formula mass, Mr for:
CuO: 63 + 16 i.e. 79
H2SO4: 1*2 + 32 + 4*16 i.e. 98.
To learn more about relative atomic mass use link below:
https://brainly.com/question/28826089
#SPJ4
One quart of liquid is equal to 0.946 Liters. Four quarts is equal to one gallon. How many Liters are equal to 10 gallons of gasoline?
Answers
Answer:
37.84 Liters
Explanation:
(see picture)

The process due to which a solid directly changes into its vapours.
Help please
Answers
Answer:
Sublimation
Explanation:
Formula equation is more informative than word equation
Answers
The formula equation includes all the required information of a compound. It has a fixed number of atoms and the amount in which they exist reacting.
What is the benefits of Formula equation?Formulas and equations permit chemists to express chemical knowledge efficiently. Both symbol and word equations exist informative. Word equation articulates the formula of the compounds. The state of all the reactants and products exists depicted in the word equation.
A word equation exists as a chemical reaction represented in words rather than chemical formulas. A word equation should express the reactants (starting materials), products (ending materials), and direction of the reaction in a state that could be used to write a chemical equation.
The formula equation includes all the required information of a compound. It has a fixed number of atoms and the amount in which they exist reacting. It furnishes a shorter path to remembering the chemicals and their usable forms.
Whereas the word equation only maintains the nature of reactants and products. It will not provide any opinion about its actual moles and weight. Therefore, formula equations exist more meaningful than word equations.
To learn more about Formula equation refer to:
https://brainly.com/question/27903246
#SPJ9