Answers
Related Questions
Calculate the pH at the equivalence point for the following titration: 0.25 M HCOOH versus 0.25 M NaOH.
Answers
The pH at the equivalence point for the titration of 0.25 M HCOOH versus 0.25 M NaOH is 8.5.
Let's consider the following neutralization reaction.
HCOOH + NaOH ⇒ HCOONa + H₂O
At the equivalence point, 0.25 M HCOOH completely reacted with 0.25 M NaOH to form 0.25 M HCOONa.
HCOONa undergoes hydrolysis. The net ionic equation is:
HCOO⁻(aq) + H₂O(l) ⇄ HCOOH(aq) + OH⁻(aq)
Given the concentration of HCOO⁻ is 0.25 M (Cb) and the basic dissociation constant of HCOO⁻ is 4.8 × 10⁻¹¹ (Kb), we can calculate the concentration of OH⁻ using the following expression.
\([OH^{-} ] = \sqrt{Kb \times Cb } = \sqrt{(4.8 \times 10^{-11} ) \times 0.25 } = 3.5 \times 10^{-6} M\)
The pOH of the solution is:
\(pOH = -log [OH^{-} ] = -log (3.5 \times 10^{-6} ) = 5.5\)
The pH of the solution is:
\(pH = 14 -pOH = 14 -5.5 = 8.5\)
The pH at the equivalence point for the titration of 0.25 M HCOOH versus 0.25 M NaOH is 8.5.
Learn more: https://brainly.com/question/2728613
How many grams of potassium permanganate contain 2.40 x 10^24 oxygen atoms?
Answers
Answer: 2.4e+24
Explanation:
It could just as easily have been selected to be 1/24th of the mass of one atom of a carbon atom, or 1/10th the mass of a calcium atom, A sample of 250 grams is equivalent to 1.58 moles of potassium permanganate.
How does an inhibitor work?A.) slows down a reaction by decreasing concentrationB.) slows down a reaction by decreasing surface areaC.) slows down a reaction by increasing activation energyD.) slows down a reaction by decreasing number of collisions
Answers
Answer
C.) slows down a reaction by increasing activation energy
Explanation
Inhibitors are compounds that modify the catalytic properties of the enzyme and, therefore, slow down the reaction rate, or in some cases, even stop the catalysis. Such inhibitors work by blocking or distorting the active site.
Inhibitors, on the contrary, increase the energy of activation of the reaction.
The correct option is
C.) slows down a reaction by increasing activation energy
Write the equation for the equilibrium constant (K) of the reaction studied in this exercise.
2C04 2- (ag) + 2Ht (ag) = CI20, 2- (ag) + H20(1)
Answers
The equation for the equilibrium constant (K) of the reaction studied in this exercise can be written as follows: K = ([\(CI_20\), 2-] * [\(H_20\)(1)]) / ([\(C0_4^ 2\)-] * [Ht])
In this equation, the concentrations of the species involved in the reaction are represented by the square brackets [ ]. The subscripts indicate the stoichiometric coefficients of each species in the balanced chemical equation.
The reaction being studied involves the following species:
\(C0_4^ 2\)- (ag) + 2Ht (ag) = \(CI_20\), 2- (ag) + \(H_20\)(1)
In the equilibrium constant expression, the concentration of \(CI_20\), 2- is multiplied by the concentration of \(H_20\)(1) and divided by the product of the concentrations of \(C0_4^ 2\)- and Ht. The stoichiometric coefficients in the balanced equation are used as exponents for the concentrations of the respective species.
It is important to note that the concentrations used in the equilibrium constant expression should be in molar units (mol/L) or expressed as partial pressures for gases.
Additionally, the equilibrium constant is specific to a given temperature, and its value provides information about the relative amounts of reactants and products at equilibrium.
For more such question on equilibrium constant visit:
https://brainly.com/question/3159758
#SPJ8
Identify the correct chemical formula. Select one: O a. K₂C₂H₂O2 0 b. K2(OH)2 O c. KCIO3 O d. 504 MATU 20 A www. wowow
Answers
Answer:
B.K2(OH)2 i think that is the answer
how many grams of carbon tetrachloride can be produced from reacting 709.0 grams of chlorine (CI2) with excess methane?
Answers
Hope this helps!
Areas of the earth with lots of direct sunlight have warm air.explain why warm air rises and leaves pockets of low pressure behind ?
Answers
Answer: Equator
Explanation:
The equator and the regions near the equator receive maximum solar radiation every year. These areas exhibit warm air. The warm air rises and the cools down then further at low pressure zone it might not be able to hold the water vapors. So the water vapors present in the air will cool down and condense in the form of clouds and the rain fall down.
give me 8 examples of addition reaction
Answers
Answer:
An addition reaction occurs when two or more reactants combine to form a single product.
Addition reactions occur with unsaturated compounds.
The general equation for an addition reaction: A+BA+B →→ C
Hydrohalogenation involves the addition of a hydrogen atom and a halogen atom to an unsaturated compound (containing a carbon-carbon double bond).
A polymer is made up of lots of smaller units called monomers. When these monomers are added together, they form a polymer. One way for polymerization to occur is through an addition reaction. Example: The polymerization of vinyl chloride monomers to form a polyvinyl chloride polymer.
Halogenation is very similar to hydrohalogenation but a diatomic halogen molecule is added across the double bond.
Hydrogenation involves adding hydrogen to an alkene. During hydrogenation the double bond is broken (as with hydrohalogenation and halogenation) and more hydrogen atoms are added to the molecule
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
which gas is used in flying balloons ?
Answers
Answer:
hydrogen
Explanation:
can i have brainliest please
The mass of the products will decrease as they absorb heat energy.
The mass of the products will increase as they absorb heat energy.
The total mass of the products will decrease as the volume increases.
The total mass of the products will equal the total mass of the reactants.
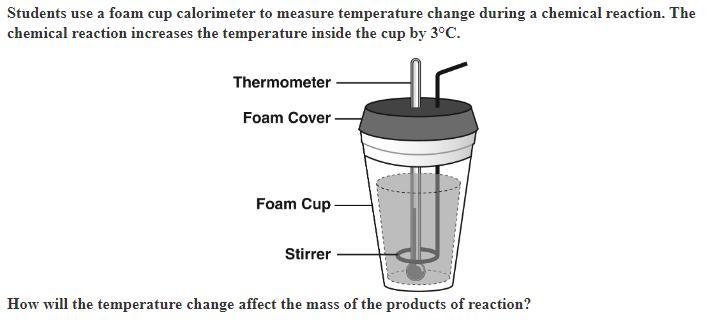
Answers
The total mass of the products will equal the total mass of the reactants.
What is the calorimeter?A calorimeter is a device used to measure the heat of chemical reactions or physical changes. It works by measuring the heat absorbed or released during a reaction or process by monitoring changes in temperature.
Calorimeters can be used to study a wide range of chemical reactions, including combustion, acid-base reactions, and reactions involving enzymes.
Calorimetry is an important technique in the study of thermodynamics, and it has many practical applications.
Learn more about calorimeter:https://brainly.com/question/28034251
#SPJ1
Draw the product formed when the Lewis acid (CH3CH2)3C reacts with the Lewis base (CH3)2NH.
Answers
Answer:
Explanation:
When the Lewis base (CH3)2NH reacts with Lewis acid Lewis acid (CH3CH2)3C⁺ which is also a carbocation , a new bond is formed as follows by the donation of a loan pair of electrons by the nitrogen atom of
(CH3)2NH . Here (CH3)2NH donates electrons so it acts as base and the second group accepts electrons so it is acid .
THe structure of the product formed is shown in the file attached .

While performing a blatant flop togain a foul called on the opposing team, LeBron James scrapes his knee and begins to bleed. His personal doctor bursts ontothe court with hydrogen peroxide (H2O2) in to clean up his wound. If the doctor applied 6.43 x 1029 molecules of hydrogen peroxide to the infected area, how many grams of hydrogen peroxide (H2O2) were used?
A)3.14 g H2O2B)1.317 x 1055g H2O2C)3.63 x 107 g H2O2D)1.138 x 1052g H2O
Answers
Answer:
i hope u find what your looking for
Determine the energy released per kilogram of fuel used.
Given MeV per reaction, calculate energy in joules per kilogram of reactants.
Consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers
1. Calculate the energy released per reaction using Einstein's famous equation E = mc^2, where E is the energy released, m is the mass defect of the reactants, and c is the speed of light. The mass defect is the difference between the mass of the reactants and the mass of the products.
2. Convert the energy released per reaction from MeV (mega-electron volts) to joules using the conversion factor 1 MeV = 1.602 × 10^-13 joules.
3. Calculate the number of reactions per kilogram of reactants. Since 1 mole of tritium plus 1 mole of deuterium is a mole of "reactions," and the total molar mass of the reactants is 5 grams, we can calculate the number of moles of reactants per kilogram.
4. Multiply the energy released per reaction by the number of reactions per kilogram of reactants to get the energy released per kilogram of fuel used.
Here's the formula:
Energy released per kilogram of fuel used = (Energy released per reaction x Conversion factor x Reactions per kilogram of reactants) / 1000
What is the density of the cube below if
the mass is 45g7
h=16m
L=5cm
W=3cm
Answers
0.1875grams/cm3 is the density of the cube below if
the mass is 45g7.
A material substance's density is its mass per unit volume. D is density, M is mass, and V is volume, therefore the formula for density is d = M/V. In terms of grammes per cubic centimeter, density is frequently expressed. As an illustration, the density of water is 1 g/cc, whereas the density of the Earth is 5.51 g/cc.
As kilos per cubic metre, density can also be calculated (in metre-kilogram-second or SI units). As an illustration, air has a density of 1.2 kilograms per cubic metre. Textbooks and manuals contain lists of the densities of common solids, liquids, and gases. The mass of a body can be calculated from its volume using density, and vice versa. The mass is equal to the volume multiplied by the density (M = Vd), whereas the volume is equal to the mass divided by the density (V = M/d). You may calculate a body's weight by multiplying its mass by the acceleration of gravity, which is typically of more practical significance than its mass.
Derive the mass and volume of this sugar cube
Mass = 45 grams
sides are h=16m
L=5cm
W=3cm
ρ = m/V
ρ = density, kg/m3, or g/(cm)3
m = mass, in kg or g
V = volume, in m3 or (cm)3
Here, ρ = density, m = mass, and v = volume.
ρ = 45/16×5×3
Density of the cube is 0.1875grams/cm3
Learn more about Density here
https://brainly.com/question/15164682
#SPJ9
Of all the Earth science careers you researched online, which three sound the most interesting to you and why?
Answers
Though this question is a matter of opinion, I can provide a possible answer to help guide you in your writing, by choosing the ecology, atmospheric science and geological fields of earth science.
What makes these fields of study interesting?These fields of study or career paths are especially interesting since they often lead to new discoveries regarding the history of our planet, or insights into the possible future of the Earth. Allow me to offer some examples:
In the field of atmospheric sciences, scientist were able to predict the warming of the earth due to greenhouse gases and give insight into a possibly grim future, allowing us to take actions to prevent it.In the field of geology, scientist were able to study the rock formations and layers of sites such as the grand canyon and provide information as to how and when such marvels of nature were formed. Finally, in the field of ecology, scientist are able to study ecosystems and the species that inhabit them, and further our understanding of interspecies relationships as well as biology.Therefore, we can conclude for the reasons listed above that these 3 career paths are some of the possible choices you can use when deciding your own three most interesting fields in Earth science.
To learn more about the Earth visit:
https://brainly.com/question/14042561?referrer=searchResults
#SPJ1
A chemical reaction was carried out by mixing 25 g of pure CaCO3 and 0.75 mole of pure HCl to give CaCl2, H2O and CO2. a. Which one is the limiting reactant and why? b. Calculate the mass of CaCl2 produced. c. How many number of water molecules are formed? d. Calculate the volume of CO2 gas liberated at STP. e. What mass of NaOH is required to absorb the whole CO2 produced in the reaction?
Answers
hola, esta pregunta es bastante difícil pero está bien, no lo sé, lo siento :) :)
All the options are solved and answer is written below
What is a Chemical Reaction ?A reaction between two or more compounds to form products made after chemical change is called a chemical reaction.
It is given that
A chemical reaction was carried out by mixing 25 g of pure CaCO₃ and 0.75 mole of pure HCl
CaCl₂ , H₂O and CO₂ are the products obtained.
CaCO₃ + 2HCl → CaCl₂ + CO₂ + H₂O
Mole ratio CaCO₃ : HCl : CaCl₂ : H₂O = 1 : 2 : 1 : 1
Molar mass of CaCO₃ = 100 g/mol
Molar mass of HCl = 36.5 g/mol
Molar mass of H₂O = 18 g/mol
Molar mass of CaCl₂= 110.98 g/mol
Moles of CaCO₃ = 25/100 = 0.25 moles
Moles of HCl present = 0.75 mole
For 0.25 moles of CaCO₃ 0.5 moles of HCl is required , as the moles of HCl is present in excess therefore
a. CaCO₃ is the limiting reactant
b. mass of CaCl₂ produced
Moles of CaCl₂ produced = 0.25 moles
1 mole means 110.98 gm
0.25 mole means 0.25* 110.98 = 27.74 gm
c. moles of water molecules formed
for 0.25 moles of CaCO₃ 0.25 moles of water will be formed
d.Volume of Co produced at STP
PV = nRT
P= 1 atm
V=?
R = 0.0821 atm L/K/mol
V = 0.25 * 0.0821 * 273 /1
V = 5.6 liter
e. The mass of NaOH required to absorb CO₂ produced in the reaction
Ratio of NaOH:CO₂ = 2 :1
0.5 moles will be required , i.e.
0.5 *40
20 grams of NaOH will be required.
To know more about Chemical Reaction
https://brainly.com/question/23919704
#SPJ2
Calculate the pH when 90.0 mL of 0.200 M HBr is mixed with 30.0 mL of 0.400 M CH₃NH₂ (Kb = 4.4 × 10⁻⁴).
Answers
The pH of the solution is 10.82.
To solve this problem, we need to determine the concentration of the hydronium ion (\(H_3O^+\)) in the solution. This can be done using the following steps:
Write the balanced chemical equation for the reaction between HBr and CH₃NH₂.
HBr + CH₃NH₂ → CH₃NH₃⁺ + Br⁻
Write the expression for the base dissociation constant (Kb) for CH₃NH₂.
Kb = [CH₃NH₃⁺][OH⁻]/[CH₃NH₂]
Calculate the concentration of hydroxide ions (OH⁻) in the solution using the Kb value and the concentration of CH₃NH₂.
Kb = [CH₃NH₃⁺][OH⁻]/[CH₃NH₂]
4.4 × 10⁻⁴ = x² / (0.400 M)
x = 6.63 × 10⁻³ M
[OH⁻] = 6.63 × 10⁻³ M
Calculate the concentration of \(H_3O^+\) using the equilibrium constant for the reaction between HBr and \(H_2O\).
\(HBr + H_2O = H_3O^+ + Br^-\)
\(Kw = [H_3O^+][OH^-] = 1.0 * 10^{-14}\\[H_3O^+] = Kw/[OH-] = 1.51 * 10^{-11}\)
Calculate the pH using the concentration of \(H_3O^+\).
\(pH = -log[H_3O^+]\\pH = -log(1.51 * 10^{-11})\)
pH = 10.82
For more question on pH click on
https://brainly.com/question/172153
#SPJ11
You add 168.90 grams of NaCl to a container and then you add 616.00 grams of water to that same container What is the weight percent of NaCl in the containerI
Answers
Answer:
The weight percent of NaCl in the container is 21.5%
Explanation:
Given that,
Mass of NaCl = 168.90 gram
Mass of water = 616.00 grams
We need to calculate the weight percent of NaCl in the container
Using formula of percentage of weight
\(weight\ \%\ of\ component\ of\ the\ solution =\dfrac{weight\ of\ the\ component\ in\ the\ solution}{total\ weight\ of\ the\ solution}\times100\)
Put the value into the formula
\(weight\ \%\ of\ component\ of\ the\ solution =\dfrac{168.90}{168.90+616.00}\times100\)
\(weight\ \%\ of\ component\ of\ the\ solution=21.5\%\)
Hence, The weight percent of NaCl in the container is 21.5%
The mass and volume of a body are 4.
4.237kg and
2.5cm 3
respectively. What is the density
Answers
Explanation:
p=mv
=(4.237)(2.5x10^-2)^3
=6.6x10^-5 kgm-3
The water in a garden hose flows at a rate of 10.0 liters per minute. What is the flow rate in gallons per hour? (1gal=3.79L)
Answers
The water in a garden hose flows at a rate of 10.0 liters per minute. Therefore, 158 gal/hr is the flow rate in gallons per hour.
What is flow rate?Flow rate is the amount of liquid that travels in a given length of time. Furthermore, the flow rate is affected by the channel through which the liquid is travelling, the area of the pipe, as well as the velocity of something like the liquid. Furthermore, the formula is. Fluid dynamic rate = pipe or channel area x liquid velocity.
The volume flow value is the rate of liquid (water flow rate formula) passing through every place in an area over time.
1 gal = 3.79 L
60 min = 1 hr
10.0 L/min x 60 min/hr x 1 gal/3.79 L = 158 gal/hr
Therefore, 158 gal/hr is the flow rate in gallons per hour.
To learn more about flow rate, here:
https://brainly.com/question/13352319
#SPJ1
If 2 g of element X combines with 7 g of element Y to form compound XY, how many grams of Y
are needed to form compound XY2?
(1 Point)
Answers
Answer:
14g of Y to form the compound XY₂
Explanation:
In the compound XY, you have 1 mole of X and 1 mole of Y. Thus, you can imagine the molar mass of X is 2g/mol and molar mass of Y is 7g/mol.
Now, in the compound XY₂, you have 1 mole of X but 2 moles of Y.
If you have 2g of X, in this case you will need:
2 moles Y * (7g / mol) =
14g of Y to form the compound XY₂
The amount of element Y needed to form compound XY2 is; 14g of element Y.
From the law of conservation of mass which states that;
Matter can never be created nor destroyed.
By stoichiometry;
Since:
7 g of element Y to form compound XY(y =1)
a g of element Y to form compound XY2(y =2)
In essence,
a g = (2 × 7g)/1
a = 14g
Therefore, 14g of element Y is needed to form compound XY2.
Read more:
https://brainly.com/question/24384921
Which bone is located between the incus and the inner ear?
cochlea
stapes
incus
malleus
Answers
Answer: The answer is incus
Suppose you have two identical 1.0 L sealed containers. Both containers are kept at exactly 25oC. One vessel contains only neon gas at 1.5 atm, and the other contains only xenon gas at 2.5 atm.
A) Is the average kinetic energy possessed by the neon atoms greater than, equal to, or less than that of the xenon atoms? Explain.
B) What variable must be changed in order to decrease the average kinetic energy of the xenon atoms?
C) Does the vessel with the xenon gas contain more, fewer, or the same number of gas particles as the vessel of neon gas? Explain.
Answers
Answer:
See explanation
Explanation:
a) The average kinetic energy of the molecules of a gas depends on the temperature and the molar mass of the gas. However, at the same temperature, all gases have the same average kinetic energy. Hence Xe and Ne atoms have the same average kinetic energy.
b) To decrease the kinetic energy of Xe atoms the temperature must be changed. When the temperature is changed, Xe a lower average kinetic energy due to its larger molar mass.
c) All gases occupying the same volume have equal number of particles at the same temperature and pressure according to Avogadro's law. Therefore, since Xe and Ne occupy the same volume at the same temperature but different pressures they do not contain the same number of particles. Xe gas contains more particles because there are more moles of Xe gas present than moles of Ne gas. The greater the number of moles of gas present, the more the number of gas particles present.
how many electrons does lead have
Answers
Answer:
The answer is 82
Explanation:
Dude in the stop says so
An unidentified compound was analyzed and found to contain 29.84 g Na, 67.49 g Cr and 72.67
g of O. Calculate the empirical formula of the compound.
Answers
Sodium dichromate is the empirical formula for the chemical.
Benzene formula: what is it?An allotrope of carbon with the chemical formula carbon 6, benzotriyne or cyclo[6]carbon is a hypothetical molecule. Six carbon atoms form the molecule's ring, which is joined by alternating triple and single bonds.
We must identify the compound's simplest whole-number atom ratio in order to obtain the empirical formula. To begin, we can convert the masses of each element to moles as follows:
moles of sodium = 29.84 g / 22.99 g/mol = 1.299 mol
moles of creatinine = 67.49 g / 52.00 g/mol = 1.298 mol
moles of oxygen = 72.67 g / 16.00 g/mol = 4.542 mol
sodium.creatinine.oxygen = 1.299 mol / 1.298 mol : 1.298 mol / 1.298 mol : 4.542 mol / 1.298 mol
sodium.creatinine.oxygen = 1.001 : 1.001 : 3.500
sodium.creatinine.oxygen = 1001 : 1001 : 3500
sodium.creatinine.oxygen = 1001/GCF : 1001/GCF : 3500/GCF
sodium.creatinine.oxygen = 143 : 143 : 500.
To know more about Sodium visit:-
https://brainly.com/question/29327783
#SPJ1
is the solid planet itself.
Answers
C 6 H 12 O 6 +6O2 = 6CO2+......
PLEASE BALANCE THIS EQUATION
ANSWER AND I WILL GIVE YOU BRAINILIEST
Answers
Answer:
2C2 H6 +7O2=4co2+6h2o+heat
Magnesium hydroxide reacts with chlorine to form magnesium chloride,
magnesium chlorate and water. How many grams of magnesium hydroxide is
needed to yield 8.00 moles of magnesium chlorate?
77.8 g Mg(OH)2
9178.1 g Mg(OH)2
2799.6 g Mg(OH)2
.823 g Mg(OH)2
How many grams of sodium sulfato pro
Answers
The grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g. None of the options provided match the calculated value of 466.64 g.
To determine the grams of magnesium hydroxide (Mg(OH)2) needed to yield 8.00 moles of magnesium chlorate (Mg(ClO3)2), we need to consider the balanced chemical equation for the reaction between magnesium hydroxide and chlorine.
The balanced equation is as follows:
2 Mg(OH)2 + 6 Cl2 → 2 Mg(ClO3)2 + 2 H2O
From the balanced equation, we can see that 2 moles of Mg(OH)2 react with 6 moles of Cl2 to produce 2 moles of Mg(ClO3)2.
Therefore, the stoichiometric ratio is 2 moles of Mg(OH)2 : 2 moles of Mg(ClO3)2.
To calculate the grams of Mg(OH)2 needed, we can use the stoichiometric ratio and the given moles of Mg(ClO3)2.
Given:
Moles of Mg(ClO3)2 = 8.00 moles
Using the stoichiometric ratio, we have:
8.00 moles Mg(ClO3)2 × (2 moles Mg(OH)2 / 2 moles Mg(ClO3)2) = 8.00 moles Mg(OH)2
To convert moles to grams, we need to multiply by the molar mass of Mg(OH)2.
The molar mass of Mg(OH)2 = (24.31 g/mol) + (2 * 16.00 g/mol) = 58.33 g/mol
Grams of Mg(OH)2 = 8.00 moles Mg(OH)2 × 58.33 g/mol = 466.64 g
Therefore, the grams of magnesium hydroxide needed to yield 8.00 moles of magnesium chlorate is approximately 466.64 g.
For more such questions on magnesium chlorate
https://brainly.com/question/12358640
#SPJ11
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.