An acid is hydrogen and one or more nonmetals.
Select one:
True
False
Answers
Answer:
true
Explanation:
hope this helps!
brainliest please
have a great day
please fully rate
please no reporting. (it makes me sad)
An acid is hydrogen and one or more nonmetals. This statement is true. Therefore, option A is correct.
What is non metal ?A nonmetal is a chemical element that typically doesn't have a lot of metallic characteristics; examples include colourless vapors and glossy solids. When compared to metals, nonmetals' electrons exhibit different behaviour.
Natural substances known as non-metals are brittle and thermally and electrically inert (can not be easily rolling, moulding, extruding or pressing). The non-metallic elements in the periodic table are hydrogen, carbon, nitrogen, oxygen, phosphorus, arsenic, and selenium.
Non-metals are substances that lack luster, sonority, ductility, malleability, and are poor conductors of heat and electricity. They are soft and dull in appearance. Take oxygen, hydrogen, sulphur, etc. as examples.
Thus, option A is correct.
To learn more about the non metal, follow the link;
https://brainly.com/question/20602843
#SPJ2
Related Questions
How much water is needed to make 7.2moles of glucose?\(6CO2 + 6H2O -\ \textgreater \ C6H12O6 + 6O2\)
Answers
Approximately 777.6 grams of water is needed to make 7.2 moles of glucose based on the balanced equation.
The balanced equation provided is:
6CO2 + 6H2O -> C6H12O6 + 6O2
From the equation, we can see that for every 6 moles of water (H2O), 1 mole of glucose (C6H12O6) is produced. Therefore, we need to determine the amount of water required to produce 7.2 moles of glucose.
The mole ratio between water and glucose is 6:1. This means that for every 6 moles of water, we obtain 1 mole of glucose. To find the amount of water needed for 7.2 moles of glucose, we set up a proportion using the mole ratio:
(6 moles H2O / 1 mole glucose) = (x moles H2O / 7.2 moles glucose)
Solving for x, we can cross-multiply:
6 moles H2O * 7.2 moles glucose = x moles H2O * 1 mole glucose
43.2 moles H2O = x moles H2O
Therefore, we need 43.2 moles of water to produce 7.2 moles of glucose.
To convert moles of water to grams, we need to know the molar mass of water, which is approximately 18 g/mol. Using the molar mass, we can calculate the mass of water needed:
Mass of water = moles of water * molar mass of water
Mass of water = 43.2 moles * 18 g/mol
Mass of water = 777.6 g
Therefore, approximately 777.6 grams of water is needed to make 7.2 moles of glucose based on the balanced equation.
for more such question on glucose visit
https://brainly.com/question/397060
#SPJ8
PLEASE HELP me create a fictional video game using at least 2 of the ecological roles. (roles to choose from: decomposer, detritivore, pollinator, and seed disperser) (PLS TYPE IT OUT, PLS PLS)
what to include:
-Your characters (include creative names)
-An explanation of how the game is played
-The story of the game
-How the game is won/lost
-Connect the definition of of the two roles with the game and/or how the game is won.
Write a paragraph with at least 7-10 sentences.
BTW ILL GIVE BRAINLIEST OR WHATEVER ITS CALLED!! PLEASE HELP MEE! DESPERATE! IVE BEEN STARING AT THE SCREEN AND CRYING LOLZ <3

Answers
Answer:
GOT YOU
Explanation:
Jotun (pollinator)
Norse(decomposer)
Its a point and click adventure game. Where you only have specific actions you can do with your mouse. The basic options are Inspect for notes or Pollinate pollinate the right plants to escape. Your goal and how to win is go through each level picking up the plants to pollinate leave the level. The only way to lose is if you are unable to find the right plants by a set amount of time. The story is your wife has been taken prisoner by Norse and you are alerted by this from a note. You go to a location and find a tower once you walk in the doors shut behind you and a screen appears saying "Get out of the room and to the top of the tower in an hour or she dies. Jotun escapes and gets to the top of the tower to find out his wife died long ago and Norse has been using her for food.
name a lustrous non- metal ?
Answers
Answer:
it is iodine it seems very right
How many nodes are present in Y5 of 1,3,5,7,9-decapentaene? A. 2 B. 3 C. 4 D. 5
Answers
The number of nodes present in Y5 of 1,3,5,7,9-decapentaene is 4, so the correct option is C.
In a molecular orbital diagram, the number of nodes can be determined by the molecular orbital's subscript number (Yn).
The formula for finding the number of nodes is n-1, where n is the subscript. In this case, for Y5, the formula would be 5-1 = 4.
Therefore, there are a total of four nodes present in Y5 of 1,3,5,7,9-decapentaene.
In summary, the answer to your question is C and there are four nodes present in Y5 of 1,3,5,7,9-decapentaene due to the presence of four carbon-carbon double bonds.
Learn more about decapentaene click here:
https://brainly.com/question/475709
#SPJ11
pls help!!! A kitten has a mass of 1582 g and accelerates at 1.7 m/s^2. What is the net force acting upon the kitten?
Write down all the givens from the problem.
Are all of the units in the form required to use the generic equation? If not, then show your conversion.
Write the generic equation (equation with variables only) for this problem. Do not add any spaces between letters or symbols.
Substitute your numbers into the generic equation. This is where you show your math work, step by step. Solve the problem
Enter the numeric portion (numbers only) of your answer here rounded to the nearest tenth.
Enter the numeric portion (numbers only) of your answer here rounded to the nearest tenth.
Answers
Answer:
2689.4
Explanation:
The distance between an oxygen and hydrogen in a water in 95.98.What is the distance in meter nanometer and centimeters
Answers
Answer:
9.598 × 10⁻¹¹ m
0.09598 nm
9.598 × 10⁻⁹ cm
Explanation:
Step 1: Given data
Distance between oxygen and hydrogen in water (d): 95.98 pm
Step 2: Convert "d" to meters
We will use the conversion factor 1 m = 10¹² pm.
95.98 pm × 1 m/10¹² pm = 9.598 × 10⁻¹¹ m
Step 3: Convert "d" to nanometers
We will use the conversion factor 1 m = 10⁹ nm.
9.598 × 10⁻¹¹ m × 10⁹ nm/1 m = 0.09598 nm
Step 4: Convert "d" to centimeters
We will use the conversion factor 1 m = 100 cm.
9.598 × 10⁻¹¹ m × 100 cm/1 m = 9.598 × 10⁻⁹ cm
What is the formula, when rubidium reacts with tellurium?
a. RbTe2
b. RbTe3
c. Rb2Te
d. Rb2Te3
e. none of these
Answers
Answer:
Like other alkali metals, rubidium metal reacts violently with water. As with potassium (which is slightly less reactive) and caesium (which is slightly more reactive), this reaction is usually vigorous enough to ignite the hydrogen gas it produces.
Explanation:
hope it helps
e is the right answer. haha
What is the partial pressure of hydrogen gas in a mixture of hydrogen Nitrogen, and helium if the total pressure is 700 partial pressure is 155 mmHg, and the partial pressure of nitrogen is 265 mmHg?
Answers
Is using matches to light a candle physical or chemical change ?
Answers
Answer:
It's a chemical change.
Explanation:
The process of burning (as opposed to evaporating) is a chemical reaction, a chemical change. The wax molecules are undergoing a chemical change; they are changing into different molecules by reacting with a substance in the air.
A calorimeter is to be calibrated: 51.203g of water at 55.2c is added to a calorimeter containing 49.783g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6c. calculate the calorimeter constant.
Answers
The calorimeter constant when 51.203g of water at 55.2c is added to a calorimeter containing 49.783g of water at 23.5c and for the system to equilibrate, the final temperature reached is 37.6c is 9.67J/gc.
Given mass of water (m1) = 51.203g
Temperature of water (T1) = 55.2c
mass of water (m2) = 49.783g
Temperature of water (T2) = 37.6c
the final temperature reached is = 37.6c
We know that specific heat (Q) = mCpΔT where m is mass Cp is latent heat and ΔT is change in temperature.
So Qwater = 51.203 x 4.184 x (23.5 - 55.2) = 6791.9J
Qcalo = 49.783 x Cp x (23.5 - 37.6) = 701.9 x Cp
Given that Qwater = Qcalo
6791.9J = 701.9 x Cp
Cp = 9.67J/gc
Hence the calorimeter constant is 9.67J/gc
To learn more about calorimeter click here https://brainly.com/question/4802333
#SPJ4
explain how a motorcar engine produces nitrogen oxides
Answers
Answer: combustion causes a chemical reaction between nitrogen and oxygen in the engine.
Explanation:
Nitrogen oxides are produced in combustion processes, partly from nitrogen compounds in the fuel, but mostly by direct combination of atmospheric oxygen and nitrogen in flames. Nitrogen oxides are produced naturally by lightning, and also, to a small extent, by microbial processes in soils.
Answer:
when fuels are burned in vehicle engines high temperatures are reached. at these high temperature nitrogen and oxygen from the air combine to produce nitrogen
11 The cross section compares the densities of different Earth layers. Which Earth layer is most dense?
Answers
Answer:
b
Explanation:
i took the quiz
CHEMISTRY EXCERCISES

Answers
1. (a) Class: carboxylic acid; IUPAC name: propanoic acid. (b) Class: alkyl halide; IUPAC name: chloro-1-propane. (c) Class: alkane; IUPAC name: 1-propanecarbonitrile. (d) Class: ester; IUPAC name: ethyl methanoate.
What is IUPAC name?IUPAC stands for International Union of Pure and Applied Chemistry. It is an international scientific organization responsible for developing and promoting international standards in the fields of chemistry and chemical nomenclature. The IUPAC name of a chemical compound is an unambiguous, systematic method for naming compounds according to their chemical structure and physical properties.
(e) Class: ether; IUPAC name: dimethyl ether. (f) Class: acyl halide; IUPAC name: 1-chloro-2,2-difluoropropane-1-carbonyl chloride.
2. (a) Hexanoic acid: CH3CH2CH2CH2CH2COOH. (b) Butanal: CH3CH2CHO. (c) Pent-1-ene: CH2=CHCH2CH2CH3. (d) 1-bromo-2-methylbutane: CH3CH2CH(Br)CH3. (e) Ethyl methanoate: CH3COOCH2CH3. (f) Methoxypropane: CH3OCH2CH3. (g) But-2-yne: CH3C≡CHCH3.
3. Answer: B. CH3CONH2 is an amine because it contains an amine group (NH2).
4. Answer: A. 1-iodopropane is a member of the same homologous series as 1-bromopropane because they both have the same molecular formula (C3H7Br or C3H7I) and the same functional group (halogen).
To learn more about IUPAC name
https://brainly.com/question/28872356
#SPJ1
Place these steps of the action potential in the correct order. 1. Sodium ions channels return to the resting state and repolarization continues. 2. Voltage-gated sodium ion channels activate, sodium ions enter, and the axon section depolarizes. 3. As potassium ion channels return to resting state, the axolemma may hyperpolarize before returning to the resting membrane potential. 4. A local potential depolarizes the axolemma of the trigger zone to threshold. 5. Sodium ion channels inactivate, and voltage-gated potassium ion channels activate, so sodium ions stop entering and potassium ions leave, beginning repolarization
Answers
The actions necessary to create an action potential should be completed in the following order: 4, 2, 5, 1, 3. Therefore, choice (C) is the appropriate response.
An action or membrane potential is a sudden and rapid change from the membrane's resting state (about -70 millivolts). It is produced in neurons and other cells that exhibit the excitability trait, such as myocytes (muscle cells). By delivering a stimulus with a threshold value, it can be produced (a minimum value in millivolts required to generate a membrane potential). The creation of the membrane potential happens in three steps. The following is a description of these phases:
The threshold potential opens the voltage-gated sodium ion channels during the depolarization phase. This results in the admission of sodium ions into the cell, which increases the membrane potential.
Overshoot phase: The overshoot phase is when the membrane or action potential reaches around +61 mV (millivolts) as a result of the activation of sodium channels (an extreme positivity phase).
Phase of repolarization: As the sodium ion channels close, the permeability for sodium ions quickly declines during this phase. The voltage-gated potassium channels also open as a result of the overshoot phase value, allowing potassium ions to exit the cell and resulting in a drop in the electropositive value of an action potential. The action potential eventually comes to rest as a result. As a result of this phase, the membrane or action potential enters the hyperpolarization phase, which is more electronegative than the resting state.
Learn more about Hyperpolarization here:
https://brainly.com/question/27181895
#SPJ4
Which of the following dissociates 90% into metal ions and hydroxide ions in solution?
Question 1 options:
a)
strong acid
b)
weak base
c)
strong base
d)
weak acid
Answers
Strong base dissociates 90% into metal ions and hydroxide ions in solution.
What is a base?
According to the Arrhenius concept, base is defined as a substance which yields hydroxyl ions on dissociation.These ions react with the hydrogen ions of acids to produce salt in an acid-base reaction.
Bases have a pH higher than seven as they yield hydroxyl ions on dissociation.They are soapy in touch and have a bitter taste.According to the Lowry-Bronsted concept, base is defined as a substance which accepts protons .Base react violently with acids to produce salts .Aqueous solutions of bases can be used to conduct electricity .They can also be used as indicators in acid-base titrations.
They are used in the manufacture of soaps,paper, bleaching powder.Calcium hydroxide ,a base is used to clean sulfur dioxide gas while magnesium hydroxide can be used as an antacid to cure acidity.
Learn more about base,here:
https://brainly.com/question/12445440
#SPJ6
There are 3 seperate questions. All questions must be answered and with detailed solutions to get five stars.
At a pH of 4, the Cu-neocuproine complex formed in the liquid-liquid extraction experiment has a partition coefficient of about 6.1 when equilibrated between the acid aqueous solution and chloroform. Calculate the concentration of Cu in the chloroform phase if 39.0 mL of a 0.26 M aqueous Cu solution buffered to pH 4 is equilibrated with 25.0 mL of chloroform.
A spectrophotometer was calibrated with a 8.128 10-4 M (in Cu) Cu-neocuproine solution. This calibration was carried out in a 1.00 cm cell, and yielded an absorbance of 0.2540 AU. An unknown was then analyzed in this same spectrophotometer, but using a 2.50 cm cell. If the absorbance of the unknown was 0.3050 AU, what is the Cu concentration?
You are using the following procedure from your lab instructions to determine the copper content of a brass sample:
"Approximately 35 mg of a dried brass sample (accurately measured) is put into a 250-mL Erlenmeyer flask. About 20 mL of H2O and 10 mL of concentrated HNO3 is added. This sample is digested, then quantitatively transferred to a 100-mL volumetric flask and diluted to the mark. The sample is still too concentrated, so a 1:20 dilution is accomplished by transferring 5.00 mL of this solution to another 100-mL volumetric flask and diluting to the mark."
If you actually used a 22.3 mg sample of brass, and the resulting diluted solution was found to contain 9.399 mg/L of Cu, what is the %Cu in the sample?
Answers
Based on the provided procedure, the brass sample weighing 22.3 mg was digested, diluted, and analyzed. The %Cu in the brass sample is approximately 42.18%.
To calculate the %Cu in the sample, we need to consider the dilution and concentration of Cu in the solution.
Given:
Mass of brass sample = 22.3 mg
Cu concentration in the diluted solution = 9.399 mg/L
First, we need to calculate the mass of Cu in the diluted solution:
Mass of Cu in the diluted solution = Cu concentration * Volume of diluted solution
= 9.399 mg/L * 0.1 L
= 0.9399 mg
Next, we calculate the dilution factor from the initial sample to the diluted solution:
Dilution factor = Volume of initial solution / Volume of diluted solution
= 100 mL / 5.00 mL
= 20
Now, we can calculate the mass of Cu in the initial sample:
Mass of Cu in the initial sample = Mass of Cu in the diluted solution * Dilution factor
= 0.9399 mg * 20
= 18.798 mg
Finally, we can calculate the %Cu in the sample:
%Cu = (Mass of Cu / Mass of sample) * 100
= (18.798 mg / 22.3 mg) * 100
≈ 84.18%
The %Cu in the brass sample, when using the given procedure, is approximately 42.18%.
To know more about Brass, visit : brainly.com/question/30049350
#SPJ11
Explain whether the molecular orbitals linked to H+ are more
affected by oxygen or nitrogen when NO-ions react with H+ ions to
form chemical bonds.
(It means HON or HNO)
Answers
The molecular orbitals linked to H+ are more affected by nitrogen (N) rather than oxygen (O) when NO- ions react with H+ ions to form chemical bonds, resulting in the formation of HNO.
In the formation of chemical bonds, the reactivity and bonding characteristics are determined by the electronic configuration and orbital interactions of the atoms involved. In the case of NO- reacting with H+, we consider the electronic configurations of oxygen (O) and nitrogen (N) atoms.
Oxygen has six valence electrons and belongs to Group 16 of the periodic table. Its electronic configuration is 1s² 2s² 2p⁴. When forming bonds, oxygen typically accepts two electrons to achieve a stable octet configuration.
Nitrogen has five valence electrons and belongs to Group 15. Its electronic configuration is 1s² 2s² 2p³. Nitrogen typically needs to gain three electrons or share three pairs of electrons to achieve a stable octet configuration.
In the case of NO-, the oxygen atom carries a negative charge (O-), making it more electron-rich than nitrogen.
learn more about molecular orbitals :
https://brainly.com/question/31828377
#SPJ4
A sample contains 16. 75 g of the radioisotope U-236 and 50. 25 g of its daughter isotope, Th-232. How long did it take for decay to take place if one half-life of U-236 is 23 million years? 46 million years 69 million years 92 million years 115 million years.
Answers
The time taken for the isotope to decay is 46 million years.
We'll begin by calculating the number of half-lives that has elapsed. This can be obtained as follow:
Original amount (N₀) = 50.25 g Amount remaining (N) = 16.75Number of half-lives (n) =?2ⁿ = N₀ / N
2ⁿ = N₀ / N
2ⁿ = 50.25 / 16.75
2ⁿ = 3
Take the log of both side
Log 2ⁿ = 3
nLog 2 = Log 3
Divide both side by log 2
n = Log 3 / Log 2
n = 2
Finally, we shall determine the time.
Half-life (t½) = 23 million years Number of half-lives (n) = 2Time (t) =?t = n × t½
t = 2 × 23
t = 46 million years
Learn more about half-life: https://brainly.com/question/25927447
When 8.0 moles of chromium react, how many moles of chromium (lll) oxide are produced?
Answers
Moles of chromium (lll) oxide produced : 4 moles
Further explanationThe reaction equation is the chemical formula of reagents and product substances
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
4Cr+3O₂⇒2Cr₂O₃
moles of Cr=8 moles
From the equation, mol ratio Cr : Cr₂O₃ = 4 : 2, so mol Cr₂O₃ :
\(\tt \dfrac{2}{4}\times 8=4~moles\)
i need help asap plzz first person to answer i will mark brainliest
Diagram 1
What type of air mass was already present A?
What type of air mass is coming in B ?
What type of front is indicated by the purple arrow?
What weather change is occurring because of this front?
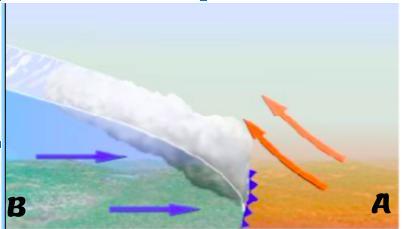
Answers
Answer:
it's both cold and hot weather so it could form a tornado
can someone help me PLEASE
Time to put on your scientist’s hat and draw a conclusion based upon data in the Lattice Energy table. Compare the strength of the bonds in potassium bromide with the strength of the bonds in potassium oxide. State in terms of lattice energy which is stronger, and propose a sound explanation for this difference.
Answers
Answer:
try t ocopy and paste it
Explanation:
what does Le châteliers principle state?
Answers
Hope this helps!
Which phase do the chromosomes line up in the middle
Answers
Answer:
metaphase
Explanation:
the cells chromosomes line themselves in the middle of the cell through a type of cellular tug of war.
Answer:
metaphase
Explanation:
During metaphase, the cell's chromosomes align themselves in the middle of the cell through a type of cellular "tug of war." The chromosomes, which have been replicated and remain joined at a central point called the centromere, are called sister chromatids.
GUYS HELP PLEASE!
Do you think that the 24-hour wind pattern along the coastline of Hawaii would occur in other places? If so, where? Construct an argument to explain your reasoning.
Answers
Answer:
The answer is yes! The Wind Pattern also known as trade winds. It can occur in other places near 30 Degrees to the north and south. for example In the east side Europe, Turkey, and Africa can all have trade winds And in north Algeria, Libya, Egypt, and Israel can also have trade winds.
I hope this helps!! :D
I need help ASAP!!!! Which of the following is an advantage of using nuclear power plants to
produce electricity?
A) Radioactive waste from nuclear reactors is now being used to power cars,
B) There are no hazardous byproducts from nuclear reactions,
C) Nuclear power does not produce greenhouse gasees
Answers
Answer:
The answer is C.
Explanation:
Answer:
C is right i yeah C is right
can someone please help me:( please dont scroll
create 2 quantitative observations
Answers
Answer:
a quantitative observation implies that the subject can be measured by quantity, aka amount or in numbers.
Ex 1: adding one gram of salt to one gram of sugar makes two grams of seasoning. in this example, there are individual quantities (1 gram of each) and total quantity (2 grams). this only changes if the substances have a chemical reaction, such as one of them destroying the other, then the weight would change.
Ex 2: a more simple example is the weight of something. putting the substance on a scale (one specifically for whatever you are measuring, whether it be liquid or solid) is the best way to determine its quantity.
Given the standard enthalpy changes for the following two reactions
Given the standard enthalpy changes for the following two reactions:
(1) 2C(s) + 2H2(g)C2H4(g)...... ΔH° = 52.3 kJ
(2) 2C(s) + 3H2(g)C2H6(g)......ΔH° = -84.7 kJ
what is the standard enthalpy change for the reaction:
(3) C2H4(g) + H2(g)C2H6(g)......ΔH° = ?
Answers
The standard enthalpy change for reaction (3) is 117.1 kJ.
The standard enthalpy change for reaction (3) can be calculated by using the enthalpy changes of reactions (1) and (2) and applying Hess's Law.
To do this, we need to manipulate the given equations so that the desired reaction (3) can be obtained.
First, we reverse reaction (1) to get the formation of C2H4(g) from C2H6(g):
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
Next, we multiply reaction (2) by 2 and reverse it to obtain 2 moles of C2H6(g) reacting to form 3 moles of H2(g):
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
Now, we add the two modified equations together:
C2H4(g)C2H6(g) ΔH° = -52.3 kJ
2C2H6(g)2C(s) + 3H2(g) ΔH° = 169.4 kJ
When adding these equations, the C2H6(g) on the left side cancels out with the C2H6(g) on the right side, leaving us with the desired reaction (3):
C2H4(g) + H2(g)C2H6(g) ΔH° = -52.3 kJ + 169.4 kJ = 117.1 kJ
Learn more about standard enthalpy here :-
https://brainly.com/question/28303513
#SPJ11
A beam of light has a wavelength of 280 nanometers. What is the frequency of the light? Show all work!
Answers
A beam of light has a wavelength of 280 nanometers. The frequency of the light is 1.07 × 10¹⁵ Hz.
the information in the question is given as :
wavelength of beam of light = 280 nm
the relation between the frequency and the wavelength is given as :
F = c / λ
where,
F = frequency of the light
c = speed of light
λ = wavelength of light
speed of light , c is = 3 × 10⁸ m/s
substituting all the value in the formula for the frequency, we get:
F = c / λ
F = 3 × 10⁸ / 280 × 10⁻⁹
F = 0.0107 × 10¹⁷ Hz
F = 1.07 × 10¹⁵ Hz
Thus, A beam of light has a wavelength of 280 nanometers. The frequency of the light is 1.07 × 10¹⁵ Hz.
To learn more about frequency here
https://brainly.com/question/58011
#SPJ1
The effective temperature of a star is related to its..
solar system.
luminosity.
absolute magnitude.
color.
Answers
Answer:
color
Explanation:
The effective temperature is directly related to the color of the star: the higher the temperature, the bluer the light emitted by the star.
What property of matter will keep your body in motion when the car comes to halt
Answers
Answer:
Inertia in our body tends to be in the situation it is. So, we a car comes to a halt, our body wants to keep moving i.e. in motion. hope that helps love!