amino acids are acids because they always possess which functional group?group of answer choicesphosphatecarboxylaminocarbonyl
Answers
E. Carboxyl. Amino acids contain a carboxyl group, which is an acidic functional group.
This group contains a COOH group, which is composed of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydrogen atom. When in solution, the carboxyl group will donate a hydrogen ion, making it acidicCarboxyl is a functional group in organic chemistry composed of a carbonyl group (C=O) with a hydroxyl group (O-H). It is found in many organic compounds such as amino acids, fatty acids, and carbohydrates. Carboxyl groups are responsible for the acids found in many organic compounds, and they can also act as a source of energy for many metabolic reactions. They are also involved in the formation of hydrogen bonds, which is essential for the formation of proteins and other biological molecules.
learn more about functional group refer:brainly.com/question/29263610
#SPJ4
complete question: Amino acids are acids because they always possess which functional group?
A. Phosphate B. Hydroxyl C. Amino D. Carbonyl E. Carboxyl
Related Questions
the ideal gas law can be related to the density of a gas by dividing both sides by v and rt and multiplying by m, the molar m. true or false
Answers
False, the ideal gas law, PV = nRT, can be related to the density of a gas by dividing both sides by RT.
The ideal gas law is given by the equation PV = nRT, where P is the pressure of the gas, V is the volume, n is the number of moles of gas, R is the universal gas constant, and T is the temperature in Kelvin.
Dividing both sides of the ideal gas law by V and RT yields:
(PV) / (VRT) = n / RT
Simplifying the left-hand side of the equation gives:
P / RT = n / V
The quantity n / V is the molar density of the gas, represented by the symbol ρ. Therefore, we can rewrite the equation as:
P / ρ = RT
Multiplying both sides of the equation by the molar mass of the gas, represented by the symbol M, gives:
(PM) / ρ = RTM
This equation relates the pressure, density, temperature, and molar mass of an ideal gas. Therefore, the statement in the question is false, as the ideal gas law is related to the density of a gas by dividing both sides by pressure and the molar density, and multiplying by the molar mass.
Click the below link, to learn more about Ideal gas equation:
https://brainly.com/question/4147359
#SPJ11"
Which statement best describes inorganic compound?
A. Inorganic compounds are compounds do that not contain carbon and hydrogen.
B. Inorganic compounds are compounds that do not contain carbon atoms only.
C. Inorganic compounds are compounds that produced by living things.
D. Inorganic compounds are compounds that contain carbon and oxygen only.
Answers
Please give brainliest I need five more.
which of the following describes correctly about the regioselectivity and stereospecificity in the bronsted acid-catalyzed addition of water to an alkene reaction? group of answer choices markovnikov orientation with syn-addition markovnikov orientation with anti-addition anti-markovnikov orientation with syn-addition anti-markovnikov orientation with anti-addition markovnikov orientation with both syn- and anti-addition
Answers
The one that best describes the regioselectivity and stereospecificity in the Bronsted acid-catalyzed addition of water to an alkene reaction is Markovnikov orientation with syn-addition. Hence option A.
The preference of E and Y for bonds with carbon atoms an or b is referred to as the Markovnikov orientation. The example below demonstrates how, depending on the reaction conditions, a proton acid HY can add to the -bond of an unsymmetrical alkene with either a Markovnikov or an anti-Markovnikov orientation.
When hydrogen itself forms a double bond with the least-substituted carbon atom in asymmetrical alkenes or alkynes, the Markownikoff or Markovnikov rule determines the orientation of the electrophilic addition of hydrogen halides. (or triple bond). So, as conculsion, option A is correct.
To know more about Markonikov orientation, visit,
https://brainly.com/question/21496002
#SPJ4
Complete question - which of the following describes correctly about the regioselectivity and stereospecificity in the bronsted acid-catalyzed addition of water to an alkene reaction?
Group of answer choices
1. markovnikov orientation with syn-addition
2. markovnikov orientation with anti-addition
3. anti-markovnikov orientation with syn-addition
4. anti-markovnikov orientation with anti-addition
5. markovnikov orientation with both syn- and anti-addition
The reaction between between common salt and concentrated tetraoxosulphate(vi) acid will liberate
A. sulphur (iv) oxide
B. oxygen and chloride
C. Hydrogen chloride gas
D. Hydrogen sulphide gas
Answers
most prolly option D
Explanation:
NaCl + H2SO4 -> Na2SO4 + HCl[g]
The reaction between between common salt and concentrated tetraoxosulphate(vi) acid will liberate Hydrogen chloride gas .
What is a Chemical reaction?
This is the type of reaction in which two or more elements/compounds react together to form a new substance.
The reaction between common salt and concentrated tetraoxosulphate(vi) acid can be seen below:
NaCl + H2SO4 ⇒ Na2SO4 + HCl(g)
Read more about Chemical reaction here https://brainly.com/question/11231920?source=archive
why is there no change in volume when pressure is applied to liquids and solids?
Answers
Liquids and solids have fixed volumes because their particles are packed tightly and have little free space to move around.
When pressure is applied, the particles in these materials are forced closer together, but because of their fixed positions, they cannot move closer to each other. The result is that the volume remains constant.
The behavior of liquids and solids is different from gases, which are compressible and have variable volumes that can change when pressure is applied. This is because the particles in a gas have much more free space to move around and can be easily compressed or expanded by pressure.
It is important to note that while the volume of a liquid or solid may not change when pressure is applied, the density of the material can change. Increasing pressure can cause the particles to become more closely packed, increasing the material's density.
Learn more about density here:
https://brainly.com/question/29775886
#SPJ4
Which acid is commonly responsible for the dissolution of limestone? Carbonic Sulfuric Nitric Hydrochloric
Answers
Answer: A: Carbonic
Explanation: Quizlet confirmed
A chemist titrates 190 ml of. 2412 nitrous acid solution with. 377 M KOH solution. Calculate the ph at equivalence. The pKa of nitrous acid is 3. 35
Answers
The equivalency solution has a pH of 2.624.
What is the procedure for making nitrous acid?Nitrous acid is frequently created by adding a mineral acid to aqueous sodium nitrite solutions. Typically, acidification is carried out at ice-cold temperatures, and HNO2 is consumed on-site. Nitrous acid in its free form is unstable and breaks down quickly.
In a neutralization process, weak nitrous acid (HNO2) reacts with strong basic KOH.
HNO2 + KOH → KNO2 + H2O
Then, we determine how many moles of KOH were used:
volume KOH x concentration equals moles KOH. KOH
moles KOH = 0.190 L x 0.377 mol/L
moles KOH = 0.07153 mol
Next, we calculate the initial concentration of HNO2:
concentration HNO2 = moles HNO2 / volume HNO2
concentration HNO2 = 0.07153 mol / 0.190 L
concentration HNO2 = 0.3765 M
[HNO2] = 0.5 x 0.3765 M
[HNO2] = 0.1883 M
The following equation can be used to model how nitrous acid dissociates in water:
HNO2 + H2O ⇌ H3O+ + NO2-
The following equation relates the pKa to the acid dissociation constant, Ka, for this reaction:
pKa = -log Ka
So we can find the Ka value from the given pKa:
pKa = -log Ka
3.35 = -log Ka
Ka = 10⁻³
Ka = 4.47 x 10⁻⁴
The relationship shown below is true for the concentrations of the species involved at equilibrium:
Ka = [H3O+][NO2-] / [HNO2]
Ka = [H3O+][NO2-] / [HNO2]
Ka = [H3O+] [HNO2]
Solving for [H3O+], we get:
[H3O+] = Ka / [HNO2]
[H3O+] = (4.47 x 10⁻⁴) / (0.1883 M)
[H3O+] = 0.002374 M
Finally, we can calculate the pH of the solution:
pH = -log[H3O+]
pH = -log(0.002374)
pH = 2.624
To know more about nitrous acid solution visit:-
https://brainly.com/question/17011556
#SPJ1
What is the role of flagella?
Answers
Answer:
a motility organelle that enables movement and chemotaxis
Explanation:
pls mark brainlist
Hope This Helps You
What volume of Kr-93 (at 25°C and 1.0 atm) is produced when 1.10 g of U-235 undergoes this fission reaction? Express your answer using two significant figures. IVO AE ? V. L
Answers
The volume of Kr-93 produced is 0.117 L
To determine the volume of Kr-93 produced when 1.10 g of U-235 undergoes fission, we need to use the ideal gas law equation:
PV = nRT
Where:
P = Pressure (1.0 atm)
V = Volume (to be determined)
n = Number of moles of Kr-93 (to be determined)
R = Ideal gas constant (0.0821 L·atm/(mol·K))
T = Temperature in Kelvin (25°C = 298 K)
First, we need to calculate the number of moles of U-235:
Given mass of U-235 = 1.10 g
Molar mass of U-235 = 235.043924 g/mol
Number of moles of U-235 = (given mass)/(molar mass)
= 1.10 g / 235.043924 g/mol
≈ 0.00468 mol
Since the fission reaction involves the same number of moles of Kr-93, we can say that the number of moles of Kr-93 produced is also 0.00468 mol.
Now, we can rearrange the ideal gas law equation to solve for V:
V = (nRT) / P
= (0.00468 mol) * (0.0821 L·atm/(mol·K)) * (298 K) / (1.0 atm)
≈ 0.117 L
Therefore, the volume of Kr-93 produced is approximately 0.117 L
Learn more about volume from the given link
https://brainly.com/question/14197390
#SPJ11
find the concentration of barium hydroxide using titration data trial

Answers
We can calculate the molarity by dividing the quantity of barium hydroxide in moles by the volume of the initial solution.
How do you find the hydroxide concentration?Implement the titration formula.The formula is molarity (M) of the acid x volume (V) of the acid = molarity (M) of the base x volume (V) of the base when the titrant and analyte have a mole ratio of 1:1.The concentration of a solution, measured in moles of solute per liter of solution, is known as its molarity.After dissociation, the concentration of barium hydroxide will be half that of the hydroxide ions because barium hydroxide dissociates into its ions when dissolved in water as:A barium hydroxide solution with a pH of 12.22 therefore has a concentration of 8.29 10 3 M.To learn more about hydroxide concentration refer
https://brainly.com/question/28464162
#SPJ1
Explain how you can find the number of neutrons in the isotope nitrogen-16
Answers
Answer:
9
Explanation:
Nitrogen-16
Atomic number of nitrogen= 7
electrons=7
protons=7
neutrons= ?
Nitrogen-'16'= 16 means the mass
16-7= 9
There are 9 neutrons in Nitrogen-16
Antarctica is the _____ largest continent in the world
Answers
Answer:
fifth
Explanation:
There is no explanation its the fifth largest
Selecting resources with reliable credentials and expertise is?
A. not important, everyone’s ideas are equally valid
B. only important when you are looking for medical advice
C. important for scientific research only
D. important for any information you look up,, on any topic
Answers
Selecting resources with reliable credentials and expertise is important for any information you look up on any topic.
What are the factors for selecting resources?There are several factors to consider when selecting resources:
Credentials: The credentials of the author or organization providing the information are important to consider. Look for information from reputable sources such as academic institutions, government agencies, and well-established organizations.
Expertise: The expertise of the author or organization is also important. Look for information from experts in the field who have relevant education and experience.
Date: The date of the information is also important. Look for the most recent information on a topic as older information may be outdated or inaccurate.
Reliability: The reliability of the source should also be considered, look for sources that are peer-reviewed, or have been validated by reputable organization
Bias: Consider the potential bias of the source and how it might affect the information presented.
Purpose: The purpose of the source should also be considered, whether it is for academic, commercial or personal use.
Accessibility: The resource should be available easily, whether is free to access or paid, and it should be in the appropriate format and language.
To know more about education, visit:
https://brainly.com/question/1602018
#SPJ1
Which tool would be the best tool to measure the volume of water absorbed by a sponge?
Answers
Answer:
The tool is called Graduated cylinders
Topic: Mass Balance. A company sells fishmeal to be used as a protein supplement in certain foods. The process consists of: a. Extraction of fish oil, stage in which a pasta is obtained that has 20% flour and 80% water. b. Drying of pasta in a rotary drum, which produces fishmeal with 40% humidity. How much pasta must be input to the process to produce 1000 kg ?
Answers
To produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta. To determine the amount of pasta required to produce 1000 kg of fishmeal, we need to consider the mass balance of the process. Let's break down the steps involved:
A. Extraction of fish oil:
The pasta obtained from the extraction stage contains 20% flour and 80% water. To calculate the amount of pasta, we need to determine the mass of flour and water in the pasta. Let's assume the total mass of the pasta is P kg.
Mass of flour = 20% of P = 0.2P kg
Mass of water = 80% of P = 0.8P kg
b. Drying of pasta:
During the drying stage, the pasta is dried in a rotary drum, resulting in fishmeal with 40% humidity. This means that the final fishmeal will contain 60% dry matter.
Let's assume the mass of the dried fishmeal is M kg.
Mass of dry matter = 60% of M = 0.6M kg
Since the dry matter in the fishmeal comes from the flour in the pasta, we can equate the mass of dry matter to the mass of flour:
0.6M kg = 0.2P kg
To produce 1000 kg of fishmeal, we want to find the corresponding value of P:
0.6M = 0.2P
P = (0.6M) / 0.2
P = 3M
Therefore, to produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta.
To know more about mass balance, click here, https://brainly.com/question/17014679
#SPJ11
The process of breaking down a larger molecule into its monomers is called.
Answers
The process of breaking down a larger molecule into its monomers is called hydrolysis. Hydrolysis is an essential chemical reaction that allows larger molecules such as polymers to be broken down into smaller, more manageable molecules called monomers.
Hydrolysis is a type of chemical reaction that uses water to break apart the bonds that hold larger molecules together, resulting in the production of monomers. Hydrolysis is the opposite of dehydration synthesis, which is the process by which monomers are joined together to form polymers.
Hydrolysis is a vital process that occurs in living organisms, allowing them to break down complex molecules such as proteins, carbohydrates, and fats into smaller molecules that can be used as energy sources or building blocks for other molecules.
To know more about breaking visit:
https://brainly.com/question/30162440
#SPJ11
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
Drag each tile to the correct location. identify the reactants and the products in this chemical equation. 2fe2o3 3c → 4fe 3co2 c co2 fe fe2o3
Answers
On the left side of the chemical process, the reactants are Fe2O3 and C. On the right side of the chemical process, the products are Fe and CO2.
Which of the following claims regarding the response listed below is true: 4Fe(s) + 3O2(g) → 2Fe2O3(g)Because the total mass of iron and oxygen in the reactants and the product are equal, the law of conservation of mass is observed.
How can reactants be recognized?In a chemical equation, the substance or substances to the left of the arrow are referred to as reactants. When a chemical reaction first begins, a substance is said to be the reactant.
Learn more about reactants here:
https://brainly.com/question/17096236
#SPJ4
PLEASE HELP ASAP, WILL GIVE BRANLIEST!!
The best practice of scientists is to label all containers while they are empty. Why do you think they do that?
Help i don't understand
Answers
Answer:so that they may not get confused in which container did they place which chemical since some chemicals are simmilar in colour
Explanation:
The scientists labeled all containers so that they will sure about which container did they put which chemical in since some of the chemicals are similar in color or some are colorless.
What is the labeling in the laboratory?Labeling in a lab is extremely important for many reasons. The laboratory is a fast-paced and efficient work zones due to proper workflow and organization.
Labeling helps to make sure that scientists are working with the correct samples or materials. Without labels, they run the danger of using the wrong chemicals or materials in experiments. This can cause experiments to fail and wastes time, resources, and funds or more than that.
Therefore, it is important for materials to work with by using labeling, as it ensures they are stored in the proper containers. The scientists label the containers to avoid confusion about the material being mixed with other materials.
Learn more about labeling in the laboratory, here:
https://brainly.com/question/28593081
#SPJ2
1. Consider the reaction: 5 A + 3 B → 2 C The rate of the reaction is found to be 0.0486 M/s. What is the rate of change of B? Be sure to consider whether the substance is disappearing or appearing in your answer.
2. For the following zero order reaction, the rate constant is 0.0311. If [A]0 = 8.68, calculate [A] at 2.9 seconds.
2 A →→ 3 B
Answers
1. Consider the reaction: 5 A + 3 B → 2 C The rate of the reaction is found to be 0.0486 M/s. Then, The rate of change of B is 0.01215 M/min.
2. For the following zero order reaction, the rate constant is 0.0311.
If [A₀] = 8.68, calculate [A] at 2.9 seconds. Then, [A] = 8.66445 ≈ 8.7.
(1) For Reaction : 5A + 3B → 2C
R₁ : 0.080486 M/s = K[A][B]² ---------------------- (1)
When half of the B has reacted, then,
⇒ R₂ = k[A][B/2] -------------------------- (2)
Dividing (2) and (1), we get:
R₂÷ R₁ = K[A] [B/2] ÷ K[A] /[B]
⇒ R₂÷ R₁ = B²/4 ÷ B²
⇒ R₂÷ R₁ = 1/4
Therefore,
R₁ /4 = 0.0486/ 4
= 0.01215 M/minute.
Therefore,
Consider the reaction: 5 A + 3 B → 2 C The rate of the reaction is found to be 0.0486 M/s. Then, The rate of change of B is 0.01215 M/min.
(2) For a Zero Order reaction:
t = K[A₀] [A] ÷ K ------------------------- (1)
Given that:
t = 2.9 Second
and, k = 0.0311
Converting Seconds into minutes:
t = 2.9 second = 0.048 Minutes ≈ 0.5 Minutes.
Putting the values in equation (1)
⇒ 0.5 = 8.68 - [A] ÷ 0.0311
⇒ 0.5 × 0.0311 = 8.68 - [A]
⇒ 0.01565 - 8.68 = - [A]
⇒ [A] = 8.66445
Therefore, [A] = 8.66445 ≈ 8.7
Based on the zero order reaction, the rate constant is 0.0311. If [A]0 = 8.68, calculate [A] at 2.9 seconds. Then, [A] = 8.7
Learn more about Reaction:
https://brainly.com/question/17434463
#SPJ4
Sodium metal and chlorine gas are produced by the electrolysis of molten sodium
chloride.
Explain why sodium chloride solution cannot be used as the electrolyte to produce
sodium metal.
[2 marks]
Answers
Sodium chloride solution cannot be used as the electrolyte to produce sodium metal because water is present in the solution, and water molecules can be electrolyzed to produce hydrogen gas, interfering with the desired production of sodium metal.
In more detail, during electrolysis, the electric current is passed through the electrolyte to facilitate the breakdown of the compound into its constituent elements. In the case of sodium chloride solution, water (H2O) molecules are also present. At the cathode, where reduction occurs, water can be electrolyzed instead of sodium ions (Na+) to produce hydrogen gas (H2) and hydroxide ions (OH-). This means that instead of obtaining sodium metal, the electrolysis of sodium chloride solution would predominantly yield hydrogen gas. To produce sodium metal, it is necessary to use molten sodium chloride, where water molecules are absent and the sodium ions can be selectively reduced to form sodium metal.
Learn more about molecules here:
https://brainly.com/question/30465503
#SPJ11
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
If a mixture is 4.6 g of water and .5 g of copper chloride ,what is the percent composition of water?
Answers
The percent composition of water : 90.2%
Further explanationThe concentration of a substance can be expressed in several quantities such as moles, percent (%) weight / volume,), molarity, molality, parts per million (ppm) or mole fraction. The concentration shows the amount of solute in a unit of the amount of solvent.
mass of water = 4.6 g
mass of Copper chloride = 0.5 g
mass of solution :
\(\tt mass~solution=mass~water+mass~Copper~chloride\\\\mass~solution=4.6+0.5\\\\mass~solution=5.1~g\)
So the percent composition of water (%mass) :
\(\tt \%water=\dfrac{mass~water}{mass~solution}\times 100\%\\\\\%water=\dfrac{4.6~g}{5.1~g}\times 100\%\\\\\%water=90.2\%\)
Copy of C.1 Compl. S middle east map q.
O d. It reacts with carbonates.
Clear my choice
of
us page
Iron (III) chloride (FeCl3) completely dissociates when dissolved in water. In a 0.60 mol/L solution of Iron (III) chloride, what
will be concentration of Cl ions?
Select one:
Oa. 1.0 mol/L
1.8 mol/L
OC. 0.20 mol/L
O d. 0.60 mol/L
Answers
The concentration of the chloride ion is obtained as 0.20 mol/L.
What is the solution concentration?We have to note that the concentration of the solution has to with the amount of the solute that we have in the system. In this case, we can see that we have the Iron (III) chloride (FeCl3) which completely dissociates when dissolved in water.
Now we are told that the concentration of the solute is 0.60 mol/L solution of Iron (III) chloride but each FeCl3 molecule produces three Cl- ions, the concentration of Cl- ions in the solution will be three times the concentration of FeCl3.
Hence;
Concentration of the chloride ion is;
0.60 mol/L/3
= 0.20 mol/L
Learn more about concentration:https://brainly.com/question/10725862
#SPJ1
The figure shows a graph of Allison’s walk to school. She follows the black vectors to get to her school. Which if the following best describes her walk?

Answers
Answer:
Allison walks 5 blocks west and 3 blocks south.
Explanation:
Vectors have both magnitude and direction. The attached figure shows the graph of Allison’s walk to school. She follows the black vectors to get to her school.
The first vector on the horizontal axis points towards west and there are 5 blocks. Initially, she walks 5 blocks in west direction.
The second vector points in the negative vertical axis. It means that she walks 3 block in south direction.
Hence, the correct option is (D) i.e. Allison walks 5 blocks west and 3 blocks south.
How many moles of magnesium is 6.02 x 1022 atoms of magnesium?
Answers
Answer: 0.0500
Explanation:
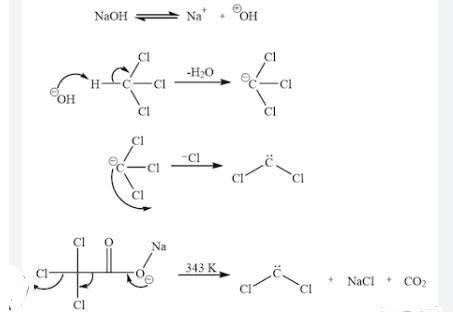
When dichlorocarbene is generated in the presence of an atkene. a dichlorocyclopropane is formed. Write the complete stepwise mechanism for the formation of dichlorocarbene, CCl2. Show all intermediate structures and show all electron flow with arrows. Draw the complete Lewis electron dot structure for dichlorocarbene, CCl2.
Answers
Dichlorocarbene is an intermediate species which is formed from the reaction of trichloromethane with a base. The intermediate CCl₃ further loss a Cl formes CCl₂.
What is dichlorocarbene?The reactive intermediate with the chemical formula CCl₂ is called dichlorocarbene. Despite not having been isolated, this chemical species is a typical intermediate in organic chemistry since it is produced from chloroform. This twisted diamagnetic molecule enters other bonds quickly.
Carbenes contains two electrons in their valence shell and they are highly reactive and therefore used in many synthetic reactions.
CHCl₃ on reaction with a strong base such as NaOH produce the intermediate anion CCl₃⁻ by the elimination of water molecule. This trichlorocarban further eliminates one Cl forms dichlorocarbene.
To find more on dichlorocarbene, refer here:
https://brainly.com/question/28202225
#SPJ4
Solution (a) has a hydrogen ion concentration of 2.7 x 10^-3 and solution (b) has a hydrogen ion concentration of 4.1 M. What are their pH and say if they are acids or bases.
Answers
Answer: a) pH = 2.56 , acidic
b) pH = 2.38, acidic
Explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
\(pH=-\log [H^+]\)
a) \([H^+]=2.7\times 10^{-3}\)
Putting in the values:
\(pH=-\log[2.7\times 10^{-3}]\)
\(pH=2.56\)
Thus as pH is less than 7, the solution is acidic.
b) \([H^+]=4.1\times 10^{-3}\)
Putting in the values:
\(pH=-\log[4.1\times 10^{-3}]\)
\(pH=2.38\)
Thus as pH is less than 7, the solution is acidic.
23. Under which conditions would carbon dioxide be most soluble in water?
O 1) 10°C and 1 atm
O2) 10°C and 2 atm
3) 20°C and 1 atm
4) 20°C and 2 atm
Answers
Answer:
20°C and 2 atm is correct
Explanation:
What is the main purpose of the second paragraph?