Air is made up of different gases, such as oxygen, nitrogen, and car
Which statement best describes these three components of air?
O Oxygen, nitrogen, and carbon dioxide are all classified as pure
O Oxygen, nitrogen, and carbon dioxide cannot react with another
Oxygen, nitrogen, and carbon dioxide are chemically bonded to
O Oxygen, nitrogen, and carbon dioxide can be classified as elemental?
Answers
The correct option would be that oxygen, nitrogen, and carbon dioxide cannot react with one another.
Why air components cannot reactThe components of atmospheric air, nitrogen, oxygen, carbon dioxide, etc., cannot react with one another because there is not enough energy in the atmosphere to set the reaction rolling.
For a reaction to take place, there must be enough energy to break the bonds in each air component. This is why the air components will not spontaneously react with one another, except during special events such as lightning and thunder.
More on air components can be found here: https://brainly.com/question/17288850
#SPJ1
Related Questions
What type of energy transfer takes place when music is coming out of a speaker?
Answers
How many centijoules of energy are required to increase the temperature of
54 grams of water 37°C?
Answers
The following equation may be used to calculate the amount of energy necessary to raise the temperature of a substance: Q = mcΔT where Q is the energy in joules.
m is the substance's mass in grams, c is the substance's specific heat capacity in J/g°C, and T is the temperature change in degrees Celsius. The specific heat capacity of water is 4.184 J/g°C. So we can calculate the energy required to raise the temperature of 54 grams of water by 37°C as follows: Q = (4.184 J/g°C) (37°C) = 6907.88 J Divide joules by 100 to get centijoules: 6907.88 J / 100 = 69.0788 cJ Using the specific heat capacity formula, we can determine how much energy is needed to raise the temperature of 54 grams of water by 37°C: Q = mcΔT where Q is the quantity of energy. m is the substance's mass, c is its specific heat capacity, and T is the temperature change.
learn more about energy here:
brainly.com/question/1932868
#SPJ4
help question 9 is due today also :)
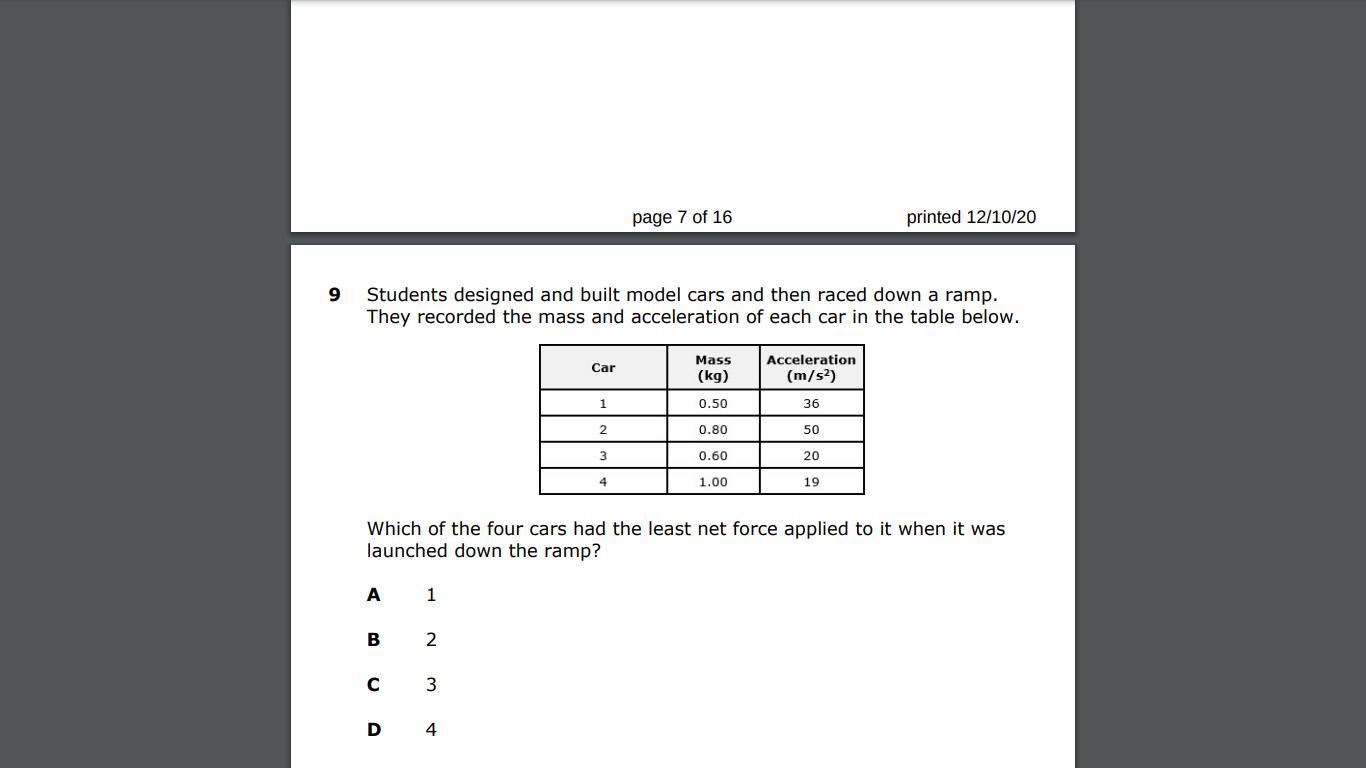
Answers
Answer:
Car 3 with a net force of 12N
Explanation:
The formula is F=MA
Hope this helps friend
Which of the following statements on HPLC modes is true? A. Increasing the polarity of the mobile phase decreases the elution time of polar compounds in normal-phase HPLC B. A non-polar stationary phase is used in normal-phase HPLC C. Compounds have a lower attraction to the mobile phase than to the stationary phase in displacement development D. A polar stationary phase is used in reversed-phase HPLC E. More polar compounds elute first in normal-phase HPLC
Answers
The following statements on HPLC modes are true is more polar compounds elute first in normal-phase HPLC (Option E).
The liquid chromatography (HPLC) is a technique in analytical chemistry employed for the separation, identification, and quantification of elements. It is considered a highly sensitive method, and it works by separating the components in a mixture with the assistance of a solvent under high pressure.
There are two modes of HPLC: Reversed-Phase HPLC (RP-HPLC) and Normal-Phase HPLC (NP-HPLC). In RP-HPLC, a nonpolar stationary phase, such as C18, is used, and polar solvents, such as water, are used as mobile phases. Polar stationary phases, such as silica gel, are used in NP-HPLC, while nonpolar solvents, such as hexane, are used as mobile phases.
More polar compounds have a greater affinity for the polar stationary phase than less polar compounds, which have a higher affinity for the nonpolar mobile phase in NP-HPLC. As a result, less polar compounds elute first in normal-phase HPLC.
Thus, the correct option is E.
Learn more about HPLC: https://brainly.com/question/13490391
#SPJ11
The term evapotranspiration combines infiltration and transpiration. group of answer choices true false
Answers
The term evapotranspiration combines infiltration and transpiration is False.
False. The term evapotranspiration does not combine infiltration and transpiration. Evapotranspiration refers to the combined process of evaporation and transpiration.
Evaporation is the process by which water changes from a liquid to a gas state and is primarily driven by heat energy from the sun. Transpiration, on the other hand, is the process by which plants release water vapor through their leaves.
Infiltration, however, is a separate process that refers to the downward movement of water from the surface into the soil. It is not directly related to evapotranspiration.
Therefore, the term evapotranspiration does not combine infiltration and transpiration.
Learn more About evapotranspiration from the given link
https://brainly.com/question/1351062
#SPJ11
the free-energy change (deltag) for the oxidation of the sugar molecules in a sheet of paper into co2 and h2o is large and negative (deltag
Answers
The oxidation of sugar molecules in a sheet of paper into CO2 and H2O is a spontaneous reaction that releases energy. The negative value of ΔG indicates that this reaction occurs naturally and is energetically favorable.
The free-energy change (ΔG) for the oxidation of sugar molecules in a sheet of paper into CO2 and H2O is large and negative (ΔG < 0). This means that the reaction is exergonic, or spontaneous, meaning it releases energy.
When sugar molecules in a sheet of paper undergo oxidation, they react with oxygen in the air to produce carbon dioxide (CO2) and water (H2O). This process is known as combustion. Combustion reactions are highly exergonic because the energy contained in the chemical bonds of the sugar molecules is released as heat and light energy.
The negative value of ΔG indicates that the products (CO2 and H2O) have a lower free energy than the reactants (sugar molecules). This means that the reaction proceeds in a direction that releases energy and is favorable under normal conditions.
To put it simply, the oxidation of sugar molecules in a sheet of paper is a reaction that naturally occurs and releases energy in the form of heat and light. This is why burning paper produces flames and ash.
It's important to note that the actual value of ΔG depends on various factors, such as the temperature and pressure. However, in general, the oxidation of sugar molecules in paper is a highly exergonic reaction.
In conclusion, the oxidation of sugar molecules in a sheet of paper into CO2 and H2O is a spontaneous reaction that releases energy. The negative value of ΔG indicates that this reaction occurs naturally and is energetically favorable.
Learn more about oxidation of sugar molecules here:-
https://brainly.com/question/15536379
#SPJ11
calculating moles of h2 gas produced from aluminum and hydrochloric acid reaction
Answers
With 0.5 moles of aluminum, 0.75 moles of hydrogen gas would be produced from the reaction.
When aluminum reacts with hydrochloric acid, it produces hydrogen gas (H₂) according to the following balanced chemical equation:
2Al + 6HCl → 2AlCl₃ + 3H₂
To calculate the moles of H₂ gas produced, you need to know the amount (in moles) of aluminum (Al) and hydrochloric acid (HCl) used in the reaction.
Let's assume you have 0.5 moles of aluminum (Al) and an excess amount of hydrochloric acid (HCl).
According to the balanced equation, the stoichiometric ratio between aluminum and hydrogen gas is 2:3. This means that for every 2 moles of aluminum, 3 moles of hydrogen gas are produced. Therefore, we can calculate the moles of hydrogen gas using the stoichiometric ratio:
0.5 moles Al × (3 moles H₂ / 2 moles Al) = 0.75 moles H₂
Learn more about stoichiometric ratio here:
https://brainly.com/question/28297916
#SPJ11
What is citric acid formula and uses?
Answers
The chemical formula for citric acid is C6H8O7. Citric acid is a weak organic acid found naturally in citrus fruits such as lemons and limes. It is also used as an ingredient in many foods, drinks, and dietary things.
Citric acid has many uses. It is used as a flavoring agent, preservative, and pH adjuster. It is also used in cleaning and laundry products, metal cleaning products, and cosmetics. In addition, it is used in pharmaceuticals and in treating kidney stones. Citric acid is also used as a chelating agent, to bind metal ions in solution, and as a sequestrant, to prevent minerals such as calcium and magnesium from forming insoluble precipitates.
To learn more about acid click here https://brainly.com/question/14072179
#SPJ4
give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of al(c2h3o2)3 and lino3 are mixed.
Answers
Since all species appear on both side of the ionic equation, there was no reaction.
Same ions present in both side of complete ionic equation are omitted to write net ionic equation. These ions are called the spectator ions.
first step - write down molecular equation as follows:
Al(C2H3O2)3(aq) + 3LiNO3 (aq) --> 3Li(C2H3O2)(aq) + Al(NO3)2(aq)
second step - write complete ionic equation;
Al3+ (aq) + 3(C2H3O2)^- (aq) + 3Li^+(aq) + 3N03^-(aq) -----> 3Li^+(aq) + 3(C2H3O2)^-(aq) + Al^3+(aq) + 3N03^-(aq)
You can see that all ions that appeared on the left hand side of the reaction equation also appeared on the right hand side of the reaction equation, thus thet are omitted. Hence there was no reaction.
Learn more about spectator ions at
Brainly.com https://brainly.com/question/22277121
#SPJ4
at what temperature would this reaction become non-spontaneous? 2C2H2(g)+5O2(g)-->4CO2(g)+2H2O(l)
Answers
We cannot determine the exact temperature at which this reaction becomes non-spontaneous without more information about ΔH and ΔS. However, we can say that the reaction is likely to become non-spontaneous at low temperatures and that the temperature at which this occurs depends on the values of ΔH and ΔS.
The spontaneity of a reaction depends on the change in Gibbs free energy (ΔG) of the system. If ΔG is negative, the reaction is spontaneous, and if ΔG is positive, the reaction is non-spontaneous. The equation for calculating ΔG is ΔG = ΔH - TΔS, where ΔH is the change in enthalpy, ΔS is the change in entropy, and T is the temperature in Kelvin.
In the case of the reaction 2C2H2(g) + 5O2(g) --> 4CO2(g) + 2H2O(l), we can determine whether the reaction is spontaneous or not at a given temperature by calculating ΔG. However, we need the values of ΔH and ΔS for this reaction, which are not provided.
Assuming that ΔH and ΔS are constant and do not change with temperature, we can still make some general statements about the spontaneity of the reaction at different temperatures.
At low temperatures, the reaction is likely to be non-spontaneous because the positive ΔS term in the ΔG equation is small compared to the negative ΔH term. As the temperature increases, the positive ΔS term becomes more significant, and the reaction becomes more spontaneous.
Eventually, at a certain temperature, the ΔG value will become positive, and the reaction will become non-spontaneous. The temperature at which this occurs depends on the values of ΔH and ΔS, which are not provided. However, we can say that the reaction will become non-spontaneous when ΔH/T is greater than ΔS.
Learn More about spontaneous here :-
https://brainly.com/question/5372689
#SPJ11
help me need an answer ASAP!!!!!!!!!!!!!!!!!!!!!!!!!!! 8th grade


Answers
The matter is composed of a large number of extremely small indivisible particles called atoms. They can neither be created nor destroyed.
What are the subatomic particles in atom?A particle which is smaller in size than an atom is defined as the subatomic particles. An atom consists of three subatomic particles. They are electrons, protons and neutrons.
The positively charged particles present in an atom is called protons whereas the negatively charged particles are electrons. The neutrons are chargeless particles.
In our daily life three balls of different colours represents a model of an atom. Here one colour denotes the protons, other two balls denotes electrons and neutrons.
A model of atom with 3 protons, 3 electrons and 4 neutrons can be represented below. It is the 'Li' atom which contains 3 protons and 4 neutrons.
Thus balls depicts a model of atom and Li atom model is shown here.
To know more about atoms, visit;
https://brainly.com/question/14214017
#SPJ1

The table shows the amount of radioactive element remaining in a sample over a period of time. radioactive decay rate amount of radioactive sample (grams) time (years) 56.0 0 47.1 400 39.6 800 33.3 1,200 28 1,600 part 1: what is the half-life of the element? explain how you determined this. part 2: how long would it take 312 g of the sample to decay to 9.75 grams? show your work or explain your answer.
Answers
1. The half life of the element, given the data from the question is 1600 years
2. The time taken for 312 g of the sample to decay to 9.75 grams is 8000 years
1. How to determine the half life of the elementHalf-life is the time taken for half a material to decay.
To determine the half life of the given element, do the following:
Original amount = 56 gHalf the original amount = 56 / 2 = 28 gTime for 28 g = 1600 yearHalf life of element = 1600 yearsHow to determine the timeWe'll begin by determining the number of half-lives that has elapsed. This can be obtained as follow:
Original amount (N₀) = 312 gAmount remaining (N) = 9.75 gNumber of half-lives (n) =?2ⁿ = N₀ / N
2ⁿ = 312 / 9.75
2ⁿ = 32
2ⁿ = 2⁵
n = 5
Finally, we shall determine the time. This can be obtained as follow:
Half-life (t½) = 1600 yearsNumber of half-lives (n) = 5Time (t) =?t = n × t½
t = 5 × 1600
t = 8000 years
Learn more about half life:
https://brainly.com/question/26374513
#SPJ1
The electron shell model of an atom has three main components the energy shell, the subshell, and the orbital. Arrange these components from the lowest to highest maximum capacity to hold electrons. The component that can hold the greatest number of electrons should be at the top.

Answers
The selection rules of quantum mechanics allow finding the result for the order of the atomic levels are:
number higher electrons -- lower number electrons
Principal > Orbital > Magnetic
n > l > \(m_l\)
The solution of the Schrodinger equation of quantum mechanics results in the energy of the in electrons in an atom that has spherical symmetry and has three constants that are related.
The constants are called quantum numbers and are: The main, the secondary or orbital and magnetic.
The principal quantum number (n) can have values from 0 to infinity
The orbital quantum number (l) can have a value from 0 to n -1, it is customary to write this number with letters
number symbol
0 s
1 p
2 d
3 f
The magnetic quantum number (\(m_l\) ) can have values from -l to l
Apart from this number there is a fourth quantum number called spin magnetic quantum number (\(m_s\)) and it can have only two values ½ and -½,
These numbers that are allowed in quantum mechanics are called select rules. We can see that in each main number (n) there can be several orbital numbers (l) and within each orbital number there can be several magnetic numbers and within each of them there is
The order of the levels from highest to lowest number of electrons are:
Spin. Lowest
Mmagnetic
Orbital
Principal Highest
In conclusion using the selection rules of quantum mechanics we can find the result for the order of the atomic levels are:
Number higher electrons -- Lower number electrons
Principal > Orbital > Magnetic
n > l > \(m_l\)
Learn more here: brainly.com/question/11855107
What is a cinder cone volcano composed of after it erupts to form a volcano?
Answers
Answer:
They are built from particles and blobs of congealed lava ejected from a single vent. As the gas-charged lava is blown violently into the air, it breaks into small fragments that solidify and fall as cinders around the vent to form a circular or oval cone.
Explanation:
hope it helps :)
List the following carbocation in each set in order from most stable to least stable: Rank from most stable to least stable. To rank items as equivalent, overlap them. What would be the major product obtained from the addition of HBr to each of the following compounds?
Answers
When HBr is added to an alkene, the major product obtained is the alkyl halide.
The specific product formed depends on the nature of the alkene and the conditions of the reaction. The reaction proceeds through electrophilic addition, where the carbocation acts as an electrophile, and the HBr molecule acts as a nucleophile.
The addition of HBr to an alkene happens through the Markovnikov addition. The nucleophilic \(Br^{-}\) ion adds to the carbon atom bearing the most hydrogen atoms, leading to the formation of an alkyl halide with the halogen (Br) attached to the more substituted carbon. This is known as the Markovnikov addition. This reaction occurs with more stable carbocations.
Learn more about Markovnikov's addition here:
https://brainly.com/question/28217404
#SPJ4

Which of the following is a characteristic property of ionic compounds? A.They have low melting points. B.They form hard, brittle crystals. C.They do not form crystals. D.They have low boiling points.
Answers
Answer:
the answer is d
Explanation:
i just did it
The characteristic property of ionic compounds is B. They form hard, brittle crystals.
Ionic bond is a chemical bond formed as the result of transfer of electrons from one atom to another.
Ionic bonds are held by strong electrostatic force. Properties of ionic bonds are:
There form crystals.they have high boiling and melting points.The are soluble in water and insoluble in solvents.They conduct electricity when dissolved in water.Find out more at: https://brainly.com/question/11148793
You are given three seismograms that recorded the same earthquake. The P and S wave arrival times are as follows:
Seismogram 1: P = 2:15pm; S = 2:18pm
Seismogram 2: P = 2:14pm; S = 2:15pm
Seismogram 3: P = 2:17pm; S = 2:21pm
Which of the following is true?
Seismogram 3 was closest to the earthquake’s epicenter.
Seismogram 2 was closest to the earthquake’s epicenter.
Seismogram 1 was farthest from the earthquake’s epicenter.
No answer text provided.
Answers
Seismogram 2 was closest to the earthquake's epicenter. The time interval between P and S waves provides an estimate of the distance from the seismograph station to the earthquake epicenter.
Smaller time intervals indicate closer proximity. In this case, Seismogram 2 has the smallest time interval of 1 minute (P = 2:14pm, S = 2:15pm), suggesting it is closer to the epicenter compared to the other seismograms. Seismogram 1 has a time interval of 3 minutes (P = 2:15pm, S = 2:18pm), indicating it is farther from the epicenter. Seismogram 3 has a time interval of 4 minutes (P = 2:17pm, S = 2:21pm), suggesting it is farther from the epicenter compared to Seismogram 2.
Learn more about Seismogram here:
https://brainly.com/question/1747723
#SPJ11
Please someone explain me this

Answers
1 and 2 are oxidation reactions.
What is an oxidation reaction?
An oxidation reaction is a type of chemical reaction that involves the transfer of electrons from one substance to another. Oxidation reactions are characterized by an increase in the oxidation state of a molecule, atom, or ion, resulting in a loss of electrons.
The substance that loses electrons is said to be oxidized, while the substance that gains electrons is said to be reduced.
Oxidation reactions play important roles in many chemical and biological processes, including energy production, metabolism, and corrosion.
Learn more about oxidation reaction:https://brainly.com/question/19528268
#SPJ1
Identify the genotypic and phenotypic ratios of offspring in the standard monohybrid cross.
Parent 1: _______________
Garden pea plant with homozygous gene pair for round seed
Garden pea plant with homozygous gene pair for wrinkled seed
Genotype (Parent 1): _______________
RR
rr
Parent 2: _______________
Garden pea plant with homozygous gene pair for round seed
Garden pea plant with heterozygous gene pair for round seed
Genotype (Parent 2): _______________
RR
Rr

Answers
The genotype and phenotype ratio for the first cross will be 100% Rr and 100% round, while the second cross will be 1RR:1Rr, 50/50 round and wrinkled respectively.
Monohybrid crossesAssuming that the seed shape allele is R(r).
For the first cross: RR x rr
Rr Rr Rr Rr
Genotype ratio = 100% Rr
Phenotype ratio = 100% round
For the second cross: RR x Rr
RR Rr RR Rr
Genotype ratio = 1RR : 1Rr
Phenotype ratio = 50% round and 20% wrinkled.
More on monohybrid crosses can be found here: https://brainly.com/question/1185199
#SPJ1
The image shows information about the element carbon as it appears in the periodic table. Based on the image, which statement is accurate? A. A carbon atom has two protons, two neutrons, and two electrons. B. A carbon atom has three protons, six neutrons, and three electrons. C. A carbon atom has six protons, six neutrons, and six electrons. D. A carbon atom has four protons, four neutrons, and four electrons.
Answers
Answer:
C. A carbon atom has six protons, six neutrons, and six electrons.
Explanation:
Carbon has a relative atomic mass of 12 and an atomic number of 6. We must recall that the atomic number of an atom is the number of protons in that atom. The atomic number is also the same as the number of electrons in the neutral atom of that element.
To ensure electrical neutrality of the atom, the number of protons in the atom must be equal to the number of electrons in the atom.
The mass number of an atom is the sum of the number of protons and neutrons present in the atom. Since carbon has a mass number of 12, then there must be six neutrons present in the atom.
According to the electronic configuration and structure of atom statement which is true is that a carbon atom has six protons, six neutrons, and six electrons.The correct option is C.
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
The carbon atom has atomic number '6' giving it six electrons and six protons and neutrons each.Thus, option 'C' is correct.
Learn more about atom,here:
https://brainly.com/question/1566330
#SPJ6
4) How many grams are in 4.63 x 102 moles of CC14?
Answers
Answer:
n=mass/molar mass
mass=?,molar mass=12+(35.5)4=154g/mol.n=4.63x10²
mass=4.63x10²x154=7128g
What is the molar mass of carbon?
Answers
65g of nitric acid are produced in a reaction. 2.5g of platinum are added to the reaction vessel at the start of the reaction to act as a catalyst. How much platinum will there be left in the vessel at the end of the reaction?
Answers
Answer:
2.5 g of platinum
Explanation:
Recall that a catalyst is a specie added to a reaction system to increase the rate of reaction. A catalyst does not participate in the chemical reaction hence it remains unchanged at the end of the chemical reaction. A catalyst merely provides an alternative reaction pathway by lowering the activation energy of the reaction system. Hence a catalysed reaction usually proceeds faster with less energy requirement than the uncatalysed reaction.
Since the catalyst does not participate in the reactions and remains unchanged at the end of the reaction, the mass of platinum will remain the same (2.5g). The mass can only change if a specie participates in the chemical reaction. Hence the answer.
2.5 g of platinum will there be left in the vessel at the end of the reaction
The following information should be considered:
Since the catalyst does not participate in the reactions and remains the same at the end of the reaction, the mass of platinum will remain the same (2.5g). The mass can only change in the case when a specie participates in the chemical reaction.learn more: https://brainly.com/question/2514933?referrer=searchResults
what are the equilibrium concentrations of cu and cl– in a saturated solution of copper(i) chloride if ksp
Answers
To determine the equilibrium concentrations of Cu and Cl- in a saturated solution of copper(I) chloride (CuCl),
We need to use the solubility product constant (Ksp) for the compound. The Ksp is an equilibrium constant that describes the extent to which a sparingly soluble compound dissolves in water.
The balanced equation for the dissociation of copper(I) chloride is as follows:
CuCl (s) ↔ Cu+ (aq) + Cl- (aq)
The Ksp expression for this equilibrium is:
Ksp = [Cu+] * [Cl-]
Now, the Ksp value for copper(I) chloride is necessary to calculate the equilibrium concentrations. However, the Ksp value is not provided in your question, and my knowledge cutoff is in September 2021, so I don't have access to the most up-to-date information. I can provide a hypothetical example to illustrate the concept, but please note that the values will not be accurate.
Let's assume the hypothetical Ksp value for copper(I) chloride is 1.0 x 10^-6. This value is purely for illustration purposes and may not reflect the actual Ksp value.
Since copper(I) chloride fully dissociates into Cu+ and Cl- ions, we can assume that the equilibrium concentration of Cu+ is equal to the concentration of Cu+ ions in the solution. Similarly, the equilibrium concentration of Cl- is equal to the concentration of Cl- ions in the solution.
Let's represent the equilibrium concentration of Cu+ as [Cu+]eq and the equilibrium concentration of Cl- as [Cl-]eq.
Now, using the Ksp expression, we can write:
Ksp = [Cu+]eq * [Cl-]eq
Let's assume that at equilibrium, [Cu+]eq = x and [Cl-]eq = y.
Therefore, Ksp = x * y
Substituting the hypothetical Ksp value, we have:
1.0 x 10^-6 = x * y
To solve for x and y, we need additional information. This could be the initial concentration of CuCl or any other relevant data. Without that information, we cannot determine the specific equilibrium concentrations of Cu+ and Cl-.
know more about copper(I) chloride (CuCl) here
https://brainly.com/question/28304953#
#SPJ11
What is the sweetest-tasting simple carbohydrate in the diet?
a. glucose
b. lactose
c. fructose
d. sucrose
e. galactose
Answers
Compared to other common sugars, The sweetest-tasting simple carbohydrate in the diet is fructose
The sweetest-tasting simple carbohydrate in the diet?A naturally occurring sugar called fructose can be found in a variety of fruits, vegetables, and honey.
Compared to other common sugars like glucose and sucrose (table sugar), it is sweeter.
Fructose is quickly absorbed into the bloodstream after consumption and can serve as a quick source of energy.
Due to its extreme sweetness, it is frequently employed as a sweetener in many processed foods and beverages.
High-fructose corn syrup is a commercial sweetener with a considerable fructose content that is frequently used in sodas and other sweetened beverages.
Therefore, fructose is the sweetest simple carbohydrate.
Option C) fructose is the correct answer.
Learn more about Fructose here: https://brainly.com/question/28117000
#SPJ4
ASAP
Compare the heat energy in a teaspoon of boiling water and a swimming pool full of room temperature water.
This is an essay question
Answers
The heat energy teaspoon of boiling water and a swimming pool full of room temperature water then swimming pool have more heat energy than the teaspoon of boiling water
Heat energy is the result of movement of tiny particles called as atom, molecule, or ion in solid liquid and gases and heat energy is the transfer from one object to the another and in Celsius scale water freezes at 0°C and boil at 100°C to 90°C however the swimming pool contains a lot more water therefore the pool has more thermal energy than the cup of tea even though the tea is hotter than the water in the pool that's why heat energy is more in the swimming pool then in teaspoon of boiling water
Know more about heat energy
https://brainly.com/question/29210982
#SPJ1
The main active ingredient or reducing agent in alkaline perms is · Glycerol monothioglycolate.
Answers
The main active ingredient or reducing agent in alkaline perms is Glycerol monothioglycolate (GMT).
alkaline perms are a type of hair treatment used to permanently change the structure of the hair. The main active ingredient or reducing agent in alkaline perms is Glycerol monothioglycolate (GMT). GMT is a chemical compound that helps break the disulfide bonds in the hair, allowing it to be reshaped and set into a new form.
GMT is commonly used in alkaline perms due to its ability to effectively reduce the disulfide bonds at a higher pH level. Alkaline perms typically have a pH level between 9 and 10, which helps to open up the hair cuticles and allow the GMT to penetrate the hair shaft.
Once the disulfide bonds are broken, the hair can be reshaped and then neutralized to reform the bonds in the new desired shape.
Learn more:About alkaline perms here:
https://brainly.com/question/30562468
#SPJ11
The main active ingredient or reducing agent in alkaline perms is actually thioglycolic acid or its derivative, ammonium thioglycolate.
It is essential to the perm process, by dissolving the disulfide bonds in the hair. The strength and natural structure of the hair is provided by these connections. Thioglycolic acid acts as a reducing agent when applied to the hair, causing disulfide bonds to be broken and the hair to be rebuilt and reshaped.
Permanent waves or curls can be created using this process. To prevent damage to the hair or scalp, it is important to handle these chemicals carefully and according to the correct safety precautions.
Learn more about Thioglycolic acid, here:
https://brainly.com/question/33450930
#SPJ4
Your question is incomplete, most probably the complete question is:
The main active ingredient or reducing agent in alkaline perms is ______________
The oxidation number of iron in the compound FeBr3 is ________.
+1
-2
+2
-1
+3
Answers
The oxidation number of iron in the compound FeBr3 is +3.
The oxidation number of an element in a compound is a measure of the charge that the element appears to have. In the compound FeBr3, Fe represents iron and Br represents bromine. To determine the oxidation number of iron in FeBr3, we need to consider the oxidation numbers of bromine and the overall charge of the compound.
In FeBr3, bromine has an oxidation number of -1. Since there are three bromine atoms in FeBr3, the total charge contributed by bromine is -3.
The overall charge of FeBr3 is neutral, which means the sum of the oxidation numbers of all the elements in the compound must be zero. Therefore, the oxidation number of iron can be calculated as follows:
Oxidation number of iron = Total charge - Charge contributed by bromine
Oxidation number of iron = 0 - (-3) = +3
Therefore, the oxidation number of iron in FeBr3 is +3.
Learn more:About oxidation number here:
https://brainly.com/question/29100691
#SPJ11
Which of the following is true about lava and magma? A Lava and magma are both types of molten rock. B Magma uses waves to break down lava over time. C When magma cools and hardens it becomes lava. D Magma is called lava when it’s below the Earth’s surface.
Answers
Answer:
a
Explanation:
magma is lava and lava is magma
A Lava and magma are both types of molten rock is true about lava and magma. Therefore, option A is correct.
What do you mean by molten rock ?Extrusive igneous rocks are formed when molten rock cools on Earth's surface. Rhyolite, pumice, and basalt are a few examples. Intrusive igneous rocks are formed when molten rock cools within Earth. Granite and gabbro are two examples.
Magma is an extremely hot liquid and semi-liquid rock found beneath the Earth's surface. The inner core, outer core, mantle, and crust make up Earth's layered structure. Magma makes up a large portion of the planet's mantle. This magma has the potential to push through holes or cracks in the crust, resulting in a volcanic eruption.
Scientists refer to molten rock underground as magma, and molten rock that breaks through the Earth's surface as lava.
Thus, option A is correct.
To learn more about the molten rock, follow the link;
https://brainly.com/question/27335589
#SPJ2
what is the coefficient for water after the equation is balanced?

Answers
Answer:
1
Explanation: