- a) write the formula unit of calcium fluoride.
b) A certain mass m of calcium fluoride contains 1.2*1023 anions. Deduce the mass (m). NA= 6.02*1023
Answers
Answer:
hope this image is helpful for you

Related Questions
Convert 300c into kelvin and Fahrenheit scale
Answers
Explanation:
300c = 1.8f+32= please calculate it yourself ☺
300c= k+273= 573k
What is the Lewis dot diagram structure for PI2F
Answers
Answer:
Please find attached the completed Lewis dot diagram structure for PI₂F
Explanation:
The number of valence electrons are;
Phosphorus = 5 Electrons
Iodine = 2 × 7 electrons = 14 electrons
Chlorine = 7 electrons
The total number of valence electrons = 14 + 7 + 5 = 26 electrons
2) We draw the symbol that represents the basic (general) structure of the molecule as follows;
The sheared electron pair are represented by single bond lines
3) We complete the octet structures round the fluorine and the iodine atoms as attached showing 18 electrons plus 6 shared electron pairs, with a maximum from step 2 to give a total of (18 + 6) 24 electron pairs
4) We add the 2 unaccounted valence electron on the phosphorus atom to give it the stable octet structure, which gives the completed Lewis structure



help asap for branliest

Answers
Answer:
4365.54 moles
Explanation:
Take the scientific notation and multiply it by the molar mass of 59.76.
Justify how the octet rule helps to predict which elements can bond with each other. Be sure to cite specific examples and non examples in
your response
Answers
preferAnswer:
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds. Atoms will react to get in the most stable state possible
Explanation:
Hopefully this is what your looking for
what is the empirical formula of a compound that has a carbon to hydrogen ratio 2-6
Answers
CH3 is the empirical formula of a compound that has a carbon to hydrogen ratio 2-6.
What is empirical formula?
The empirical formula of a chemical compound is the simplest and most basic ratio of the constituent elements present in it. It is written as the smallest whole-number ratio of the constituent elements, and does not indicate the amount or concentration of each element.
The empirical formula of a compound is the simplest whole number ratio of atoms in a molecule. In this case, the given carbon to hydrogen ratio of the compound is 2:6, which can be simplified to 1:3. This means that for every one carbon atom, there are three hydrogen atoms in the compound.
6H / 2C × 1/2 / 1/2 → 3 H / 1C
Therefore, the empirical formula of this compound is CH3.
To learn more about empirical formula the link
https://brainly.com/question/14044066
#SPJ1
in the krebs citric acid cycle, how much of the original carbonyl carbon from acetyl-coa will remain in oxaloacetate after two full cycles?
Answers
None, it will all be lost as Carbon dioxide.
Krebs or citric acid cycle is also known as The tricarboxylic acid (TCA) cycle.
The main source of energy for cells and an important part of aerobic respiration. Aerobic respiration is the respiration occurs in the presence of oxygen.
This cycle harnesses the available chemical energy of the acetyl coenzyme A (acetyl CoA) into the reducing power of nicotinamide adenine dinucleotide (NADH).
TCA cycle metabolizes acetate that is derived from carbohydrates, proteins, and fats to form adenosine triphosphate (ATP) which is the body's energy currency.
To know more about TCA cycle:
brainly.com/question/15561070
#SPJ4
Using the rules for significant figures, what do you get when you multiply 67.6 by 1.2?
Answers
Answer:
The answer should have as many significant figures as the lowest number used in the multiplication, which is 1.2 .
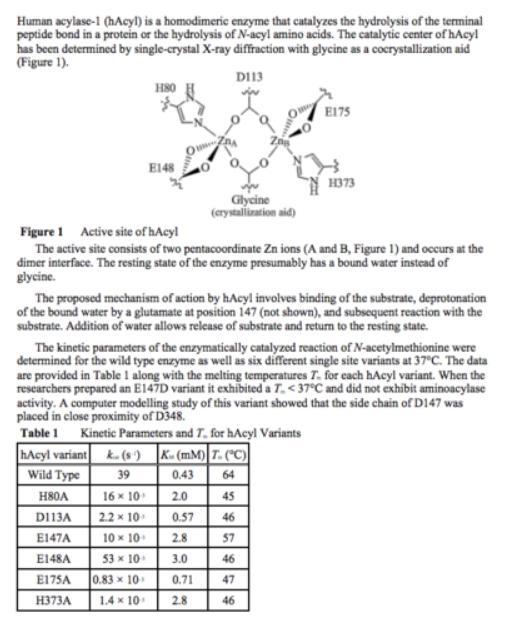
why does the proposed mechanism involve deprotonation prior to reaction? deprotonation as described in the passage causes the reacting:
Answers
Deprotonation as described in the attached passage causes the reactive -OH to attack the substrate. The correct option is C.
Deprotonation of the reacting water creates it more nucleophilic and reactive towards the electrophilic carbonyl atom of the amide group.
What is deprotonation?Deprotonation is the opposite of protonation. Protonation is the addition of proton (H+) to an atom, ion or molecule. In the other side, deprotonation is the removal of proton from any atom, ion or molecule.
Deprotonation can be a significant step in a chemical reaction. Acid–base reactions generally happen faster than any other step that may determine the product of a reaction. For example, deprotonation of an alcohol forms the negatively charged alkoxide, that is a much stronger nucleophile.
Learn more about deprotonation at: https://brainly.com/question/5628309
#SPJ4
Although part of your question is missing, you might be referring to this full question: Why does the proposed mechanism involve deprotonation prior to reaction? Deprotonation as described in the passage causes the reacting:
A. The reactive amine group to attack the substrate
B. The reactive carbonyl group to attack the substrate
C. The reactive -OH to attack the substrate
D. The reactive zinc ion to attack the substrate
Which forces can be represented in a free body diagram
Answers
Make and use a table: to compare mitosis and meiosis in humans. Vertical headings should include: What Type of Cell (Body or Sex), Beginning Cell (Haploid or Diploid), Number of Cells Produced, End-Product Cell (Haploid or Diploid), and Number of Chromosomes in New Cells.
Answers
Replicated chromosomes split into two new nuclei during the mitotic cell cycle phase, which is referred to in cell biology. With a fixed number of chromosomes, genetically identical cells are produced during the cell division process known as mitosis. As a result, mitosis is also known as equational division.
Meiosis is a unique form of germ cell division that creates gametes, such as sperm or egg cells, in sexually reproducing animals. In the end, four cells with just one copy of each chromosome are produced after two rounds of division.
Meiosis creates cells that are genetically distinct from the parent and have half as much DNA as mitosis, which divides a single "parent" cell into two genetically identical "daughter" cells. Most cells in the body go through mitosis on a regular basis, however some do so more frequently than others.
Meiosis results in genetic variety because of crossing over and independent assortment while mitosis produces identical cells for the mother cell and each daughter cell. Meiosis produces cells with half as many chromosomes as the mother cell, while mitosis produces nuclei with the same amount as the mother cell.
To know more about mitosis and meiosis, click on the link below:
https://brainly.com/question/6842108
#SPJ9
How many grams of CaSO4 would be produced if 200 grams of Fe2O3 reacted
Answers
As a result, we would anticipate 487.49 grams of Calcium sulfate to result from a reaction between 200 grams of Iron and Calcium sulfate.
How many kilos does one molecule weigh?Number-wise, the mass of one mole (or formula unit) in atomic mass units is equal to the mass of one mole (or formula unit) in grams. One mole of Oxygen molecules, for instance, weighs 32.00 g and a single Oxygen molecule, 32.00 u.
We can use the following chemical equation, assuming you meant to inquire about the interaction between Iron and Calcium sulfate:
Iron + Calcium sulfate → Ferrous sulfate + Calcium
These numbers can be used to determine how many moles of iron there are in 200 grams:
200 g Iron × (1 mol Iron / 55.85 g Iron) = 3.58 mol Iron
We can infer that 3.58 moles of Calcium sulfate would be formed in this reaction because the stoichiometric ratio of Iron to Calcium sulfate is 1:1.
We can use the following equation to determine the mass of Calcium sulfate generated:
Mass of Calcium sulfate= number of moles of Calcium sulfate× molar mass of Calcium sulfate
Mass of Calcium sulfate = 3.58 mol Calcium sulfate × 136.14 g/mol
Mass of Calcium sulfate = 487.49 g
To know more about molecules visit:-
https://brainly.com/question/1446104
#SPJ9
Write the chemical formula for the following: hydroxide
Answers
Answer: OH−.
Explanation: Hydroxide, any chemical compound containing one or more groups, each comprising one atom each of oxygen and hydrogen bonded together and functioning as the negatively charged ion OH-.
A very large tank initially contains 100 L of pure water. Starting at time t=0 a solution with a salt concentration of 0.5 kg/L is added at a rate of 6 L/min. The solution is kept thoroughly mixed and is drained from the tank at a rate of 4 L/min. Answer the following questions. 1. Let y(t) be the amount of salt (in kilograms) in the tank after t minutes. What differential equation does y satisfy? Use the variable y for y(t). Answer (in kilograms per minute):
dt
dy
= 2. How much salt is in the tank after 20 minutes? Answer (in kilograms)
Answers
After 20 minutes, there would be 0.75 kilograms of salt in the tank.
1. Let's derive the differential equation that y(t) satisfies. The rate at which salt is added to the tank is given by the concentration of the solution (0.5 kg/L) multiplied by the rate at which the solution is added (6 L/min). The rate at which salt is drained from the tank is given by the concentration of salt in the tank (y(t) kg/L) multiplied by the rate at which the solution is drained (4 L/min). Therefore, the differential equation is:
dy/dt = (0.5 kg/L * 6 L/min) - (y(t) kg/L * 4 L/min)
Simplifying further, we have:
dy/dt = 3 - 4y(t)
2. To find the amount of salt in the tank after 20 minutes, we can solve the differential equation. One approach is to find the particular solution by assuming y(t) takes the form of a constant, y. Substituting this into the differential equation, we have:
dy/dt = 3 - 4y
Setting dy/dt to zero (since y is constant), we can solve for y:
0 = 3 - 4y
4y = 3
y = 3/4
y = 0.75 kg
Therefore, after 20 minutes, there would be 0.75 kilograms of salt in the tank.
Learn more about constant https://brainly.com/question/27983400
#SPJ11
Should your height be measured in meters or centimeters
Answers
Your height should be measured in meters. Centimeters is much too small of a unit to measure the several feet most people are.
Pure gold has a density of 19.3 g/cm^3 . How large would a piece of gold be if it
had a mass of 318.97 g?
Answers
Density = Mass/Volume but it can also be rearranged to:
Volume = Mass/Density
Given in the question:
Mass - 318.97 g
Density - 19.3 g/cm3
Calculation
Density = Mass/Volume
= 318.97/19.3
= 16.52 \(cm^{3}\)
Therefore, the volume of the gold is 16.52 \(cm^{3}\)
Why can different numbers of metal and monkeys atoms create ionic bonds together?
Answers
Answer:
i'm assuming you meant to say nonmetal atoms... in an ionic bond, electrons from the metal are transferred to the nonmetal. The reason why different numbers of metal and nonmetal atoms can combine is so their charges cancel out. take CaCl2 for example. There are two chlorine atoms for every calcium atom. If it was just CaCl, the charges wouldn't cancel out since calcium has a charge of 2+ and chlorine has a charge of 1-. Therefore to balance the compound, you need two chlorines.
complete the electron‑pushing mechanism for the reaction of the γ‑hydroxyaldehyde in hydrochloric acid by adding any missing atoms, bonds, charges, nonbonding electrons, and curved arrows. note the use of a generic alcohol representing another alcohol molecule in solution.
Answers
The mechanism for the reaction of gamma-hydroxyaldehyde in hydrochloric acid can be explained in terms of electron-pushing.
The missing atoms, bonds, charges, nonbonding electrons, and curved arrows can be added to complete the mechanism.
Below is the complete electron-pushing mechanism for the reaction:
Step 1: The lone pair of electrons on the oxygen atom of the gamma-hydroxyaldehyde molecule attacks the hydrogen ion from the hydrochloric acid to form a dative bond between the oxygen atom and the hydrogen ion.
The resulting product is an oxonium ion.
Step 2: The oxygen atom of the oxonium ion donates its lone pair of electrons to the carbon atom attached to the hydroxy group.
This causes the formation of a double bond between the carbon and oxygen atoms, and at the same time, the alcohol molecule represented by ROH acts as a nucleophile and donates its lone pair of electrons to the oxonium ion to form a bond. This generates an intermediate.
Step 3: The electrons from the C-H bond attached to the gamma carbon shift towards the oxygen atom, and the oxygen atom donates its electrons to form a double bond between the carbon and oxygen atoms.
This causes the formation of a carbonyl group.
The intermediate formed in the second step is converted to the product of the reaction.
Step 4: The electron from the C-H bond attached to the beta carbon shifts towards the carbon atom, and the bond between the carbon atom and the oxygen atom breaks to form a double bond. This results in the formation of an none product.
Note that curved arrows indicate the movement of electrons.
The curved arrow originating from an electron-rich site and pointing towards an electron-poor site represents the donation of a pair of electrons.
Similarly, the curved arrow originating from an electron-poor site and pointing towards an electron-rich site represents the acceptance of a pair of electrons.
To know more about gamma-hydroxyaldehyde visit;
https://brainly.com/question/31984913
#SPJ11
Find the concentration of calcium ion (in ppm) in a 3.97 g pill that contains 42.2 mg of Ca2+. Enter to 0 decimal places.
Answers
The concentration of calcium ions is 10629 ppm.
The concentration of calcium ions in parts per million (ppm) in the given pill can be calculated as shown below.
concentration in ppm = (mass of solute/mass of solution) x \(10^6\)
Here, the mass of the solute is given as 42.2 mg of Ca2+, and the mass of the solution is given as 3.97 g. We first need to convert the mass of the solute to grams:
mass of solute = 42.2 mg = 0.0422 g
Substitute these values into the above formula.
concentration in ppm = (0.0422 g / 3.97 g) x \(10^6\) = 10629.7 ppm
Rounding off to 0 decimal places as required, the concentration of calcium ions in the given pill is 10629 ppm.
To learn about concentration:
https://brainly.com/question/29710767
#SPJ4
the same
3. If the values for both mass and volume double,
the value for density will
Answers

When most liquids freeze, explain what happens to the motion and the space between the atoms.
Answers
Answer:
atoms relative motion slow down and begin to vibrate in place
I’m so tired and don’t wanna do school…

Answers
But nobody wants to be in school tho :(
The graph shows the speed of a car
traveling east over a 12 second
period. Based on the information in
the graph, it can be concluded that
in the first 6 seconds, the car is -
A changing its direction.
B experiencing less and less
friction.
moving at a constant speed
D climbing a steep incline.

Answers
Answer:
Letter B.
Explanation:
Changing its direction and climbing a steep incline can't be proven only by the graph info. You can see that it's speed isn't constant in the graph.
We have letter B left.
calculate the volume of a stock solution of ba(oh)2, with a concentration of 0.10 m, needed to prepare 8.0 l of 0.072 m ba(oh)2.
Answers
5.76 L of stock solution is needed to prepare 8L of 0.072 M of 0.10M concentration of Ba (OH)2. This is calculated by the expression of Molarity.
Molarity is a measure of the concentration of a chemical species in particular of a solute in a solution in terms of amount of substance per unit volume of solution. It is also known as the Molar concentration. By using Molarity equation,
M1 V1 = M2 V2
The volume of a stock solution of Ba(oh)2, with a concentration of 0.10 m, needed to prepare 8.0 l of 0.072 m Ba(oh)2. A stock solution is prepared by weighing out an appropriate portion of a pure solid or by measuring out an appropriate volume of a pure liquid placing it in a suitable flask and diluting to a known volume.
M1 = 0.10M
M2 = 0.072M
v2 = 8L
We can calculate for V1 by putting all these values in the expression of Molarity.
V1 = M2 V2 / M1
= 0.072 M * 8L / 0.10 M
= 5.76 L
To learn more about Molarity please visit:
https://brainly.com/question/26873446
#SPJ4
what is the wavelength range of photons that produce 40-kev electrons in compton scattering?
Answers
The wavelength range of photons that produce 40-keV electrons in Compton scattering is essentially the same as the initial wavelength of the photons.
In Compton scattering, a photon interacts with an electron, transferring some of its energy and momentum to the electron. The change in energy of the photon is related to the scattering angle through the Compton wavelength shift equation:
Δλ = λ' - λ = h / (mec) * (1 - cos(θ))
where Δλ is the change in wavelength, λ' is the scattered wavelength, λ is the initial wavelength, h is the Planck constant, me is the electron mass, c is the speed of light, and θ is the scattering angle. Given that the energy of the electron is 40 keV, use the equation for the energy of a photon to determine the initial photon energy:
E = hc / λ
40,000 eV = (hc / λ') - (hc / λ)
Simplifying the equation:
hc / λ' = (hc / λ) + 40,000 eV
λ' = (λ * λ') / (λ - λ')
Substituting λ' = λ - Δλ, we get:
λ - Δλ = (λ * (λ - Δλ)) / λ
Simplifying further:
λ - Δλ = λ - Δλ
This equation indicates that the change in wavelength is negligible compared to the initial wavelength.
Learn more about Compton scattering here:
https://brainly.com/question/29306626
#SPJ11
If we start with 20 atoms of plutonium – 239, how many would remain after 48,240 years?
Answers
The amount of atoms of plutonium – 239 that would remain after 48240 years is 5 atoms
How to determine the number of half-lives Half-life (t½) = 24100 yearsTime (t) = 48240 yearsNumber of half-lives (n) =?n = t / t½
n = 48240 / 24100
n = 2
How to determine the amount remaining Original amount (N₀) = 20 atomsNumber of half-lives (n) = 2Amount remaining (N) = ?N = N₀ / 2ⁿ
N = 20 / 2²
N = 20 / 4
N = 5 atoms
Learn more about half life:
https://brainly.com/question/26374513
#SPJ1
A concept map for four types of intermolecular forces and a certain type of bond is shown.
An ellipse is shown. Inside the ellipse is written Are ions present. An arrow from the right side of the ellipse has Yes written on it and points to another ellipse. This ellipse has Are polar molecules present written inside it. This ellipse has two arrows coming out of it. One arrow has Yes written on it and leads to a rectangular box that has D written inside it. The second arrow has No written on it and leads to a rectangular box that has E written inside it. An arrow from the left of the topmost ellipse has No written on it and leads to an ellipse that has Are molecules with permanent dipoles present Written on it. An arrow that has No written on it, points from this ellipse towards a rectangle that has A written inside it. An arrow that has Yes written on it, points from this ellipse towards another ellipse that has Is H atom bonded to F, O, or N atom Written on it. This ellipse also has two arrows coming from it. The arrow with Yes written on it leads to a rectangle that has C written on it and the arrow that has No written on it leads to a rectangle that has B written inside it.
Which of the following correctly identifies the intermolecular force represented by D and compares its strength relative to the intermolecular force represented by C?
D represents ion-dipole forces, which are weaker than the force represented by C.
D represents hydrogen bonding, which is weaker than the force represented by C.
D represents ion-dipole forces, which are stronger than the force represented by C.
D represents hydrogen bonding, which is stronger than the force represented by C.
Answers
The concept map describes the different types of intermolecular forces and their relationships with each other.
Starting from the top ellipse, if ions are present, then we move to the next ellipse where we ask if polar molecules are present. If polar molecules are present, we then ask if the molecules have permanent dipoles. If they do not have permanent dipoles, then we identify the intermolecular force as London dispersion forces (represented by A). If they do have permanent dipoles, then we ask if there is a hydrogen bond present. If a hydrogen bond is present, we identify the intermolecular force as hydrogen bonding (represented by C). If there is no hydrogen bond present, we identify the intermolecular force as dipole-dipole forces (represented by B) or ion-dipole forces (represented by D), depending on whether ions are present or not.
Therefore, D represents ion-dipole forces, which are stronger than the force represented by C.
In general, ion-dipole forces are stronger than hydrogen bonding because ions have much stronger charges than polar molecules.
\(\begin{align}\huge\colorbox{black}{\textcolor{yellow}{I hope this helps !}}\end{align}\)
\(\begin{align}\colorbox{purple}{\textcolor{lime}{Please mark as brillinest !}}\end{align}\)
\(\textcolor{cyan}{\small\textit{If you have any further questions, feel free to ask!}}\)
predict the formula of francium phosphide
Answers
The chemical formula for francium phosphide is FrP.
What is a chemical formula?A chemical formula is described as a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus and minus signs.
There are three main types of chemical formulas which include :
empirical formula molecular formula and structural formula .Learn more about chemical formulas at: https://brainly.com/question/11574373
#SPJ1
Primary succession is most likely caused by?
Answers
Answer:
volcanic eruption.
mark my answer as brainlest......
How much heat is needed to condense 22. 25 grams of nitrogen gas at â€""195. 8°C? The latent heat of vaporization of nitrogen is 199. 0 J/g. Joules.
Answers
The heat energy needed to condense 22.25 grams of nitrogen gas at -195.8°C can be calculated as follows: 4390.125 Joules.
Latent heat is the energy released or absorbed by a material during a change of state (phase change) without causing any temperature change. When a gas changes to a liquid state, the process is called condensation. Thus, the given problem is related to the calculation of the heat needed to condense nitrogen gas at -195.8°C.According to the question, Amount of nitrogen gas = 22.25 grams Latent heat of vaporization of nitrogen = 199.0 J/g As we know that latent heat is the energy required for a substance to change its state without a change in temperature.
The energy required to condense 22.25 grams of nitrogen gas can be determined as follows: Heat energy = (Amount of nitrogen gas) × (Latent heat of vaporization of nitrogen)Heat energy = 22.25 g × 199.0 J/g Heat energy = 4390.125 Joules Thus, the heat energy needed to condense 22.25 grams of nitrogen gas at -195.8°C is 4390.125 Joules.
To know more about nitrogen visit:
https://brainly.com/question/16711904
#SPJ11
you have a stock solution labeled 0.278 m silver nitrate. how many milliliters are needed to make 15.0 ml of 0.0750 m silver nitrate?
Answers
As an antimicrobial, silver nitrate, an organic compound, is used. Silver nitrate is used topically (to also be applied to a skin) to cauterise sick tissues around a skin wound.
How should silver nitrate be applied to a wound?Dip its caustic tip of a potassium iodide applicator stick into deionized or distilled water to slightly moisten it. Along the tissue that needs to be cauterised, rub and turn the applicator tip. Never use the tip to contact any other parts of the body.
How should silver nitrate be applied to a wound?Dip its caustic tip of a potassium iodide applicator stick into deionized or distilled water to slightly moisten it. Along the tissue that needs to be cauterised, rub and turn the applicator tip. Never use the tip to contact any other parts of the body.
To know more about antimicrobial visit:
https://brainly.com/question/29312754
#SPJ1