A typical level of sodium in blood is 3.24 mg/mL. What is the millimolar concentration of sodium in blood?
Answers
Answer:
black lives dont matter
Explanation:
Answer:
sorry you have to get the answer yourself without using the internet.
regards,
Weill
Explanation:
Related Questions
If 5.4 moles of Fe react with 4.7 moles of O2, what is the maximum amount of Fe2O3 (in moles) that can be produced? What is the limiting reactant?
a
3.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
b
2.7 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.
c
7.1 moles of Fe2O3 is the maximum amount that can be produced. Oxygen is the limiting reactant.
d
10.8 moles of Fe2O3 is the maximum amount that can be produced. Iron is the limiting reactant.
Answers
Answer:
2.7 moles of Fe₂O₃ is the maximum amount that can be produced. Iron is the limiting reactant.
Explanation:
The balanced reaction is:
4 Fe + 3 O₂ → 2 Fe₂O₃
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of each compound participate in the reaction:
Fe: 4 molesO₂: 3 molesFe₂O3: 2 molesThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
You can use a simple rule of three as follows: if by stoichiometry 4 moles of Fe reacts with 3 moles of O₂, how much moles of Fe will be needed if 4.7 moles of O₂ react?
\(moles of Fe =\frac{4. moles of Fe*4.7 moles of O_{2}}{3 moles of O_{2} }\)
moles of O₂= 6.27
But 6.27 moles of Fe are not available, 5.4 moles are available. Since you have less moles than you need to react with 4.7 moles of O₂, iron Fe will be the limiting reagent.
So you can use a simple rule of three as follows: if by stoichiometry 4 moles of Fe produce 2 moles of Fe₂O₃, how many moles of Fe₂O₃ will be produced if 5.4 moles of Fe react?
\(moles of Fe_{2}O_{3}=\frac{5.4 moles of Fe*2 moles of Fe_{2} O_{3} }{4 moles of Fe}\)
moles of Fe₂O₃= 2.7 moles
Then:
2.7 moles of Fe₂O₃ is the maximum amount that can be produced. Iron is the limiting reactant.
Which statements describe inorganic compounds?
Answers
Answer:
Inorganic compounds is typically chemical compound that lacks carbon hydrogen bonds.
Explanation:
Inorganic compounds most that contain carbon considered inorganic, that is study of inorganic compounds is known as inorganic chemistry.Inorganic compounds does not necessarily mean that it does not occur within living thing,inorganic and organic chemistry is merely semantic.Inorganic compounds are the four types of compounds like :- water, acids, salts and bases.Inorganic compounds most of the although the compositions of the deep remain active area of investigation.Inorganic compounds majority of its content deals with metal complexes of organic compound.Inorganic compound define inorganic polymer as the skeletal structure that does not include carbon atoms.Inorganic compounds is a substance that does not contain both carbon and hydrogen,inorganic compounds do contain hydrogen atoms.Inorganic compounds are held together process extremely high melting and boiling points.The products of the chlor-alkali process, Cl₂ and NaOH, are kept separated.(b) ClO⁻ or ClO₃⁻ may form by disproportionation of Cl₂ in basic solution. What determines which product forms?
Answers
To produce ClO⁻ and ClO₃⁻, the mole ratio of Cl₂ is 1 :2 and 1 : 2.
What is Disproportionate reaction ?The reaction for disproportionate of Cl₂ in basic medium to produce ClO⁻ and ClO₃⁻ is:
Cl₂ (g) + 2OH⁻ (aq) → Cl⁻(aq) + ClO⁻ (aq) + H₂O(l)
3Cl₂ (g) + 6OH⁻ (aq) → 5Cl⁻ (aq) + ClO₃⁻ (aq) + 3H₂O (l)
When ratio of Cl₂ is 1: 2 then ClO⁻ is produced. Ratio is 3 : 6 then ClO₃⁻ is produced.
To produce ClO⁻ ; the mole ratio of Cl₂
= \(\frac{1}{2}\)
= 1 : 2
To produces ClO₃⁻ ; mole ratio of Cl₂
= \(\frac{3}{6}\)
= 1 : 2
Thus from the above conclusion we can say that To produce ClO⁻ and ClO₃⁻, the mole ratio of Cl₂ is 1 :2 and 1 : 2.
Learn more about the Chlor-Alkali Process here: https://brainly.com/question/11945425
#SPJ4
what does Le châteliers principle state?
Answers
Hope this helps!
only Q4a, pls help i cant process this question
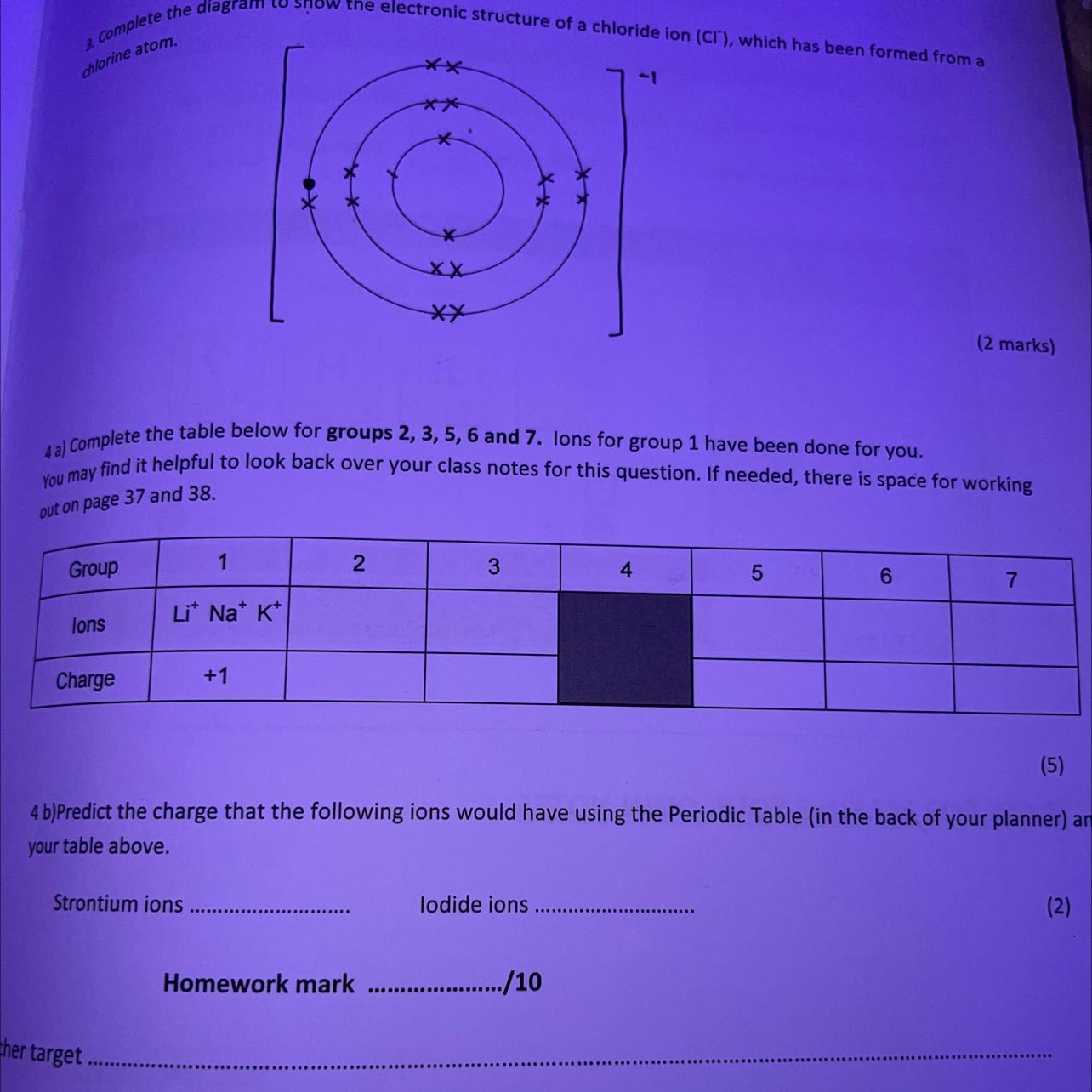
Answers
Explanation:
The group 2 ions are in the second column in the periodic table, for example Be, Mg and Ca
The group 3 ions are in the third column in the periodic table, for example B, Al and Ga
It's the same thing for the rest of the groups
Ions either lose or gain electrons to have a full outer shell, just like the noble gases
metal atoms lose electrons from their outer shell to form positively charged ions
From groups 1 to 3 the charge is the same as the group number
Non metals gain electrons on their outer shell to form negatively charged ions
From groups 5 to 7 the charge is 8 subtract the group number
Example: what is the charge for group 6 ions?
8 - 6 = 2, and the charge is negative so 2-

The molar mass is the mass of which of the following? (check all that apply)the mass of a substance per molethe average atomic mass unitsthe g/mol of a substance6.0221 x 10^23 particles of a substance
Answers
The molar mass is the mass of which of the following?
The mass of a substance per mole and the g/mol of a substance is correct.
The molar mass of a substance is the mass per mole of its entities (molecules, atoms, ions, etc)
6.0221x10^23 particles of a substance is also correct. The mole contains 6.022 x 1023 entities. The number of entities in a mole is called Avogadro's number.
Answer:
The mass of a substance per mole
The g/mol of a substance
6.0221 x 10^23 particles of a substance
draw the skeletal (line-bond) structure of (r)-1-ethyl-3-ethynylcyclohex-1-ene. use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, where applicable.
Answers
The skeletal line-bond structure of (r)-1-ethyl-3-ethynyl-cyclohex-1-ene is as follows:
A disubstituted cyclohexene molecule contains an ethyl group on C1 and an ethynyl group on C3.
To represent the dash or wedge bond structure we have to first identify the asymmetric centers in the molecule.
An organic compound's skeletal structure was made up of a group of atoms that also are joined together to form the compound's fundamental structure. Chains, branches, and/or rings of bound atoms can make up the skeleton. Heteroatoms were skeletal atoms that are not carbon as well as hydrogen.
The asymmetric center in the cyclohexene molecule is C-3 in the cyclohexene so the dash and wedge structure can also be shown as follows:
To know more about (r)-1-ethyl-3-ethynyl-cyclohex-1-ene , click here
https://brainly.com/question/2400746
#SPJ4


if i am using a propane canister in a hot air balloon burner to take off on a typical morning flight at the albuquerque balloon festival, what is the temperature change the propane undergoes as it is throttled from its saturation pressure to the ambient pressure in the burner? is it gas?
Answers
The temperature changes of the propane as it is throttled from its saturation pressure to the ambient pressure depend on the specific condition and the pressure changes that are involved. However, propane is gas indeed.
Saturation pressure is the pressure at which a substance changes phase into a gas phase at a specific temperature. In other words, it is the pressure at which vaporization starts to occur to the substance. When a substance is heated at constant pressure, its temperature will increase until it reaches a boiling point and begin to vaporize. At this point, the saturation pressure is reached, further increasing the temperature.
For propane, the saturation pressure when the temperature is 20°C is around 8.5 atm.
Learn more about propane at https://brainly.com/question/9472786
#SPJ4
Use the chart to determine which pair of atoms has the greatest difference in electronegativity.
A. Ca – Cl
B. H – Cl
C. Fe – Cl
D. P – Cl
Answers
The electronegativity for chlorine is 3.2. Now, we must look for the electronegativity that has the greatest difference from chlorine.
Calcium (Ca) - 1.0
Hydrogen (H) - 2.2
Iron (Fe) - 1.8
Phosphorus (P) - 2.2
As you can see, the element with the lowest electronegativity level is Calcium, therefore the greatest difference in electronegativity would be present between Calcium and Chlorine.
What volume of 8.25 M NaOH solution must be diluted to prepare 2.40 L of 0.500 M NaOH solution? 0.356L 145 mL
39.6L 438 ml
Answers
To prepare 2.40 L of 0.500 M NaOH solution from an 8.25 M NaOH solution, you need to determine the volume of the concentrated solution required.
You can use the dilution equation:
M1 × V1 = M2 × V2
where M1 is the initial molarity, V1 is the initial volume, M2 is the final molarity, and V2 is the final volume.
Here, M1 = 8.25 M, M2 = 0.500 M, and V2 = 2.40 L. You need to solve for V1:
8.25 M × V1 = 0.500 M × 2.40 L
Rearrange the equation to find V1:
V1 = (0.500 M × 2.40 L) / 8.25 M
V1 = 1.20 L / 8.25
V1 ≈ 0.145 L or 145 mL
So, to prepare 2.40 L of 0.500 M NaOH solution, you must dilute 145 mL of 8.25 M NaOH solution.
To learn more about molarity here
https://brainly.com/question/2817451
#SPJ11
Un gas a una temperatura de 38°C, tienen un volumen de 21 Lts Litros. ¿Qué volumen tendrá si la temperatura sube a 67°C?.
Answers
Answer:
. ¿Qué
Explanation:
Hope you have a great day
A 390 ml of aqueous solution prepared by dissolving 0.6 g of aluminum nitrate al(no3)3 in water, the concentration of nitrate ion (no3-) is __________m. the molar mass of al(no3)3 is 213 g/mol
Answers
The concentration of the nitrate ion, NO₃¯ in the solution given the data is 0.0216 M
What is molarity?This is defined as the mole of solute per unit litre of solution. Mathematically, it can be expressed as:
Molarity = mole / Volume
How to determine the mole of Al(NO₃)₃Mass of Al(NO₃)₃ = 0.6 g Molar mass of solute = 213 g/mol Mole of Al(NO₃)₃ =?Mole = mass / molar mass
Mole of Al(NO₃)₃ = 0.6 / 213
Mole of Al(NO₃)₃ = 0.0028 mole
How to determine the molarity of Al(NO₃)₃Mole of Al(NO₃)₃ = 0.0028 mole Volume = 390 mL = 390 / 1000 = 0.39 L Molarity of Al(NO₃)₃=?Molarity = mole / Volume
Molarity of Al(NO₃)₃ = 0.0028 / 0.39
Molarity of Al(NO₃)₃ = 0.0072 M
How to determine the molarity of NO₃¯Dissociation equation
Al(NO₃)₃(aq) <=> Al³⁺(aq) + 3NO₃¯(aq)
From the balanced equation above,
1 mole of Al(NO₃)₃ contains 3 moles of NO₃¯
Therefore,
0.0072 M Al(NO₃)₃ will contain = 0.0072 × 3 = 0.0216 M NO₃¯
Thus, the molarity of NO₃¯ in the solution is 0.0216 M
Learn more about molarity:
https://brainly.com/question/15370276
#SPJ4
What is the approximate
Hrxn for the hydrogen combustion reaction given the following bond energies?
O-H 470 kJ/mole, H - H 430 kJ/mole, O=O 500 kJ/mole. 2H2(g) + O2(g) --> 2H2O(g)
Answers
The approximate Hrxn for the hydrogen combustion reaction can be +520 kJ/mol.
To calculate the approximate Hrxn for the given reaction, we need to determine the energy required to break the bonds in the reactants and the energy released when new bonds are formed in the products.
Reactants;
2 H-H bonds (in 2 H₂ molecules) = 2 x 430 kJ/mol
1 O=O bond (in 1 O₂ molecule) = 1 x 500 kJ/mol
Total energy required to break bonds in reactants = (2 x 430 kJ/mol) + (1 x 500 kJ/mol) = 1360 kJ/mol
Products;
4 O-H bonds (in 2 H₂O molecules) = 4 x 470 kJ/mol
Total energy released when new bonds are formed in products = (4 x 470 kJ/mol) = 1880 kJ/mol
Therefore, the approximate Hrxn for the hydrogen combustion reaction can be calculated as follows;
Hrxn = energy required to break bonds in reactants - energy released when new bonds are formed in products
= -1360 kJ/mol + 1880 kJ/mol
= +520 kJ/mol
Since the value of Hrxn is positive, this indicates that the reaction will be endothermic.
To know more about combustion reaction here
https://brainly.com/question/12172040
#SPJ4
Determine the molecular geometry IBr−4 and make a sketch
Answers
IBr4 has square planar geometry and its hybridization is sp3d2.
Iodine and bromine make up the two different halogen atoms that make up the inter-halogen compound IBr4-. It only partially dissolves in benzene and ether. The central Iodine atom of the IBr4-Lewis structure, which has a -1 formal charge and is covalently bonded to four Bromine atoms, is present. The IBr4-Lewis structure has a square planar shape. The central iodine atom is surrounded by two lone pairs of electrons on either side, four bonding electron pairs on one plane, and four bonding electron pairs arranged in a square pattern. This molecule has the generic formula AX4E2 according to VSEPR theory.Sp3d2 hybridization is present in the IBr4-molecule. One electron from the central iodine atom's 5s and 5p orbitals is promoted to the 5d orbital, where it combines with another electron to form a 6 sp3d2 hybrid orbital with the same energy. Each sp3d2 hybrid orbital combines with a bromine atom's 4p orbital to form an I-Br bond.
The attached image shows structure of IBr-4
To learn more about hybridization click here:
https://brainly.com/question/6986666
#SPJ4

In the reaction __H2 + N2 → 2 NH3, what coefficient should be placed in front of H2 to balance the reaction?
Answers
Answer:
3
Explanation:
the product of the equation includes 6H....so because there are already 2 hydrogens in the reactants, if you have three of those, or 3*2=6....therefore the coefficient should be 3
A teacher did an experiment to show the movement of particles in solids, liquids, and gases. The experiment is shown below. The teacher pulled the string attached to the stretched balloon, first slowly. Then fast and finally vigorously
Answers
Answer:
This question is incomplete. However, the completed question is below
A teacher did an experiment to show the movement of particles in solids, liquids, and gases. The experimental set-up is shown below: An inverted glass jar is shown. Small circles representing foam balls are shown inside the jar. A stretched balloon is shown tied at the mouth of the jar. A string is shown drawn out from the middle of the stretched balloon. A hand is shown pulling the drawn out string. The teacher pulled the string attached to the stretched balloon, first slowly, then fast, and finally vigorously. Which of the following is most likely correct about the pulling of the string and the corresponding state of matter being represented? (a) Gas is represented when pulled fast, solid when pulled slowly, liquid when pulled vigorously. (b) Gas is represented when pulled slowly, liquid when pulled fast, solid when pulled vigorously. (c) Solid is represented when pulled slowly, liquid when pulled fast, gas when pulled vigorously. (d) Solid is represented when pulled vigorously, liquid when pulled fast, gas when pulled slowly.
The correct answer is C
Explanation:
These question has to do with the "freedom" of particles in each state of matter. In solids, the particles are compact and do not move freely within the solid molecule hence the reason for the definite shape of solid. The particles of liquids move more freely than solid but not as much as gases, hence the reason for there free flowing nature. Particles of gases move more fast and rapidly; colliding against one another and the wall of the container.
From the above description, it can be deduced that to illustrate these particles the teacher will have to represent the particles of the solid as been moved slowly, particles of the liquid will be pulled fast and that of gas will be pulled vigorously using balloons. Thus, the correct option is C
Answer:
the correct answer is c!
Explanation:
Solid is represented when pulled slowly, liquid when pulled fast, gas when pulled vigorously.
How is a sodium ion symbol written?
Na+
So+
ООО
SO-
Na-
Answers
Answer:
Na^+
Explanation:
The symbol for sodium is Na. The term "sodium ion" assumes that the reader knows that sodium's single 3s electron is susceptible to theft by any nearby element that has a high electron affinity. Sodium's ionization energy is low, allowing the 3s electron to move elsewhere and leave behinf a positively charged Na^+ atom.
The decomposition of 3.08 g nahco3 yields 1.04 g na2co3. what is the percent yield of this reaction?
a. nahco3(s)
b. na2co3(s)
c. co2(g)
d. h2o(g)
Answers
The percent yield for each compound:
a) For NaHCO3: 100%
b) For Na2CO3: 126.2%
c) For CO2: 100%
d) For H2O: 0%
To find the percent yield, we first need to determine the theoretical yield and actual yield for each compound. The theoretical yield is the amount of product that would be obtained if the reaction proceeded perfectly, while the actual yield is the amount of product obtained in reality.
Let's calculate the theoretical yield for each compound:
a) NaHCO3(s): Since 3.08 g of NaHCO3 is given, the theoretical yield of NaHCO3 would also be 3.08 g.
b) Na2CO3(s): The given problem states that 1.04 g of Na2CO3 is obtained. However, since Na2CO3 is formed from NaHCO3, we need to consider the molar mass ratio between NaHCO3 and Na2CO3. The molar mass of NaHCO3 is 84 g/mol, and the molar mass of Na2CO3 is 106 g/mol. Using this ratio, we can calculate the theoretical yield of Na2CO3:
(1.04 g Na2CO3) × (84 g NaHCO3 / 106 g Na2CO3) = 0.824 g NaHCO3
c) CO2(g): CO2 is produced during the decomposition of NaHCO3, and it is a gas. Therefore, we need to convert the mass of NaHCO3 to moles and then use the balanced chemical equation to find the moles of CO2 produced. The balanced equation for the decomposition of NaHCO3 is:
2 NaHCO3(s) -> Na2CO3(s) + CO2(g) + H2O(g)
The molar mass of NaHCO3 is 84 g/mol.
(3.08 g NaHCO3) / (84 g/mol NaHCO3) = 0.0367 mol NaHCO3
According to the balanced equation, 1 mole of NaHCO3 produces 1 mole of CO2. Therefore, the theoretical yield of CO2 is also 0.0367 mol.
d) H2O(g): Similarly, we can use the balanced equation to determine the theoretical yield of water. According to the equation, 1 mole of NaHCO3 produces 1 mole of H2O. Therefore, the theoretical yield of H2O is 0.0367 mol.
Now, let's calculate the percent yield for each compound:
Percent yield = (Actual yield / Theoretical yield) × 100
a) For NaHCO3:
Percent yield = (3.08 g / 3.08 g) × 100 = 100%
b) For Na2CO3:
Percent yield = (1.04 g / 0.824 g) × 100 = 126.2%
c) For CO2:
Percent yield = (0.0367 mol / 0.0367 mol) × 100 = 100%
d) For H2O:
Percent yield = (0 mol / 0.0367 mol) × 100 = 0%
To summarize, the percent yield for NaHCO3 is 100%, for Na2CO3 is 126.2%, for CO2 is 100%, and for H2O is 0%.
Learn more about molar mass:
https://brainly.com/question/837939
#SPJ11
help pls this is physical science (9th grade )

Answers
Answer:
mamamkalalllaamakkaoakmaa
decide how to supersets these substances? If if is not possible, please write "cannot be separated" on the space provided

Answers
Answer: (although the question does not sate whether if you separate them physically or through energy. so i did both)
1. can be separated (When high-energy ultraviolet rays strike ordinary oxygen molecules (O2), they split the molecule into two single oxygen atoms, known as atomic oxygen)
2. can be separated, but through electrolysis, fiscally moving a crane to generate electricity to separate the molecules
3. Most solid particles, composed of diamagnetic or weak paramagnetic materials, cannot be extracted by a conventional magnetic separator. physically cannot be separated. but through heat yes
4. but there is a catch: doing so requires energy. ... If energy from coal were applied to drive the decomposition reaction, more CO2 would be released than consumed, because no process is perfectly efficient. so it cant be separated physically
5. it can be separated but it needs energy physically cannot be separated.
Explanation:
Hey please answer this thanks.

Answers
Explanation:
Percentage composition of oxygen = (80/134) * 100% = 59.7%.
difference between metals and non-metals with the reference to :
A) Number of electrons in outer or valent shell
B) Formation of cation and anion
C) Reaction with dilute action
Answers
Answer:
C) Reaction with dilute action
Explanation:
Metals are good conductors of electricity and heat.
Non-metals are insulators that don't allow heat and electricity to pass through them. Hence, non-metals are bad conductors of electricity and heat.
Base your answer to the following question on the information below and on your knowledge of
chemistry.
Wood is mainly cellulose, a polymer produced by plants. One use of wood is as a fuel in
campfires, fireplaces, and wood furnaces. The molecules of cellulose are long chains of repeating
units. Each unit of the chain can be represented as CHI1oOs. The balanced equation below
represents a reaction that occurs when CoH1oOs is burned in air.
C6H1100, + 602 + 6C02 + 5H120 + heat
Show a numerical setup for calculating the percent composition by mass of carbon in C6H100s
(gram-formula mass = 162. 1 g/mol)
Answers
To calculate the percent composition by mass of carbon in \(C_6H_{100}\), we need to determine the molar mass of carbon in the compound and divide it by the molar mass of the entire compound.
The balanced equation given indicates that for every 1 mole of \(C_6H_{100}\) burned, 6 moles of \(CO_2\) are produced. Using the molar masses of carbon and \(C_6H_{100}\) , we can calculate the percent composition by mass of carbon.
The molar mass of carbon (C) is 12.01 g/mol. The molar mass of \(C_6H_{100}\) can be calculated as follows:
(6 × 12.01 g/mol) + (1 × 1.01 g/mol) + (10 × 16.00 g/mol) = 162.10 g/mol.
To determine the percent composition by mass of carbon in \(C_6H_{100}\) , we need to find the mass of carbon in 1 mole of \(C_6H_{100}\) . Since there are 6 carbon atoms in the compound, the mass of carbon is:
(6 × 12.01 g/mol) = 72.06 g.
Now we can calculate the percent composition by mass of carbon:
(72.06 g / 162.10 g) × 100% ≈ 44.5%.
Therefore, the percent composition by mass of carbon in \(C_6H_{100}\) is approximately 44.5%.
To learn more about molar mass refer:
https://brainly.com/question/837939
#SPJ11
Suppose, in the near future, the problems with creating energy through nuclear fusion reactions are overcome. What
are some of the advantages of using a fusion reactor to produce electricity?
Answers
Answer:
Nuclear Fusion reactions power the Sun and other stars. In a fusion reaction, two light nuclei merge to form a single heavier nucleus.
Explanation:
HELP ASAP
If a steel nail is wrapped 8 times with an insulated copper wire and each end of is attached to a 1.5 V single-A battery we find that it will lift the weight of 2 paper clips. Hypothesis: If 8 more coils are added to the nail we will be able to pick up twice as many paper clips. When we ran the experiment, we observed and recorded that when we added 8 more wraps around the nail (16 coils) we could lift 4 paper clips and when we added 8 more wraps (24 coils) we could lift 6 paper clips. Which of the following is/are true?
I. Something went wrong with the experiment since it didn't give us the results we expected.
II. The hypothesis was supported with the results of the experiment.
III. It can be concluded that every time 8 wraps are added to the nail, the total number of paper clips lifted doubles.
II and III only
II only
I only
I and III only
Answers
Based on the relationship between the number of turns of the coils and the strength of an electromagnet, the true options are:
The hypothesis was supported by the results of the experiment:
The correct option is II only.
What is the relationship between the number of turns of the coils and the strength of an electromagnet?The strength of the electromagnet rises with the number of turns in the coil.
This is due to the fact that as the coil "cuts" through the magnetic field, the sum of the individual emf s created by each turn increases with the number of coils turns.
The relative speed between the coil and magnet, as well as the magnet's strength, are additional elements that influence the strength of the induced current in a dynamo in addition to the number of coils. The intensity of the generated current increases with magnet strength and relative speed.
Learn more about electromagnets at: https://brainly.com/question/17231807
#SPJ1
The photoelectron spectrum for the element nitrogen is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the ato
f the atom?
A. The leftmost peak represents the valence electrons.
B. The two peaks at the right represent a total of three electrons.
C. The electrons in the ls sublevel have the smallest binding energy
D. The electrons in the 2p sublevel have the smallest binding energy

Answers
Answer:
D. Is the correct option.
Explanation:
2p level contains the electrons furthest from the nucleus in the case of Nitrogen thus it's much easier to disperse/remove the electrons from the shell due to low pull of nucleus energy.
The best explanation of how the spectrum is consistent with the electron shell model of the atom is the electrons in the 2p sub level have the smallest binding energy. Thus, option D is correct.
What is photoelectron spectrum?
Photoemission spectroscopy also known as photoelectron spectroscopy which photoelectric effect is the process in which electron are get energy from external source of energy like sunlight deals with energy measurement of energy emission or electrons emitted from solids, gases or liquids by the process of photoelectric effect,
Photoelectric effect is the process in which electron are get energy from external source of energy like sunlight and get excited and comes in excited state from the ground state due to this process continuos flow of electron is take place and after that flow of energy is takes place.
Therefore,The best explanation of how the spectrum is consistent with the electron shell model of the atom is the electrons in the 2p sub level have the smallest binding energy. Thus, option D is correct.
Learn more about electrons here:
https://brainly.com/question/1255220
#SPJ2
What are the free moving charged particles in a Carbon electrode made of electrode
Answers
The free moving charged particles in a Carbon electrode made of electrode are electrons.
An electrode is a substance that conducts electricity, which means it allows electric charges to travel through it. During electrolysis, an electrode is used to provide an electric current for the reduction and oxidation reactions that take place.
A carbon electrode is a type of electrode that is made of carbon. Carbon electrodes are commonly used in batteries and fuel cells because they are lightweight and can easily conduct electricity.
Electrons are free moving charged particles in a carbon electrode made of electrode. Electrons are negatively charged subatomic particles that orbit the nucleus of an atom. They are found in the outer shells of atoms and can move freely from one atom to another when they are excited by an electric current.
When an electric current is passed through a carbon electrode, the electrons in the outer shells of the carbon atoms are excited and become free moving charged particles. This allows the carbon electrode to conduct electricity and to participate in reduction and oxidation reactions during electrolysis.
For more such questions on electrode, click on:
https://brainly.com/question/28302450
#SPJ11
the principal astronomical alignment at stonehenge, as well as the most common astronomical alignment at archaeoastronomical sites worldwide:______
Answers
Principal astronomical alignment at Stonehenge: Summer solstice sunrise. Most common astronomical alignment at archaeoastronomical sites worldwide: Equinox sunrise and sunset alignments.
The main astronomical alignment at Stonehenge is the alignment of the central axis with the rising sun during the summer solstice. During this event, the sun rises precisely over the Heel Stone, a large upright stone located outside the main circle of stones. This alignment is believed to have held great significance for the builders of Stonehenge, as it marked the longest day of the year and held cultural and ceremonial importance. Stonehenge's layout and design were carefully constructed to align with celestial events, and the summer solstice alignment is one of the most prominent and well-known features of the site. Most common astronomical alignment at archaeoastronomical sites worldwide: Equinox sunrise and sunset alignments.
learn more about astronomical here:
https://brainly.com/question/1764951
#SPJ11
A beekeeper found that when stung by a bee, application of a little solution of sodium hydrogen carbonate helped to relieve the irritation of the affected area.Explain
Answers
Answer:
A bee sting releases an acid. When sodium hydrogen carbonate is applied, it neutralizes the acid since it's a base.
Explanation:
The sting of an bee contains formic acid. When an bee stings, it injects the acidic liquid into the skin, similar to ants. The effect of the sting can be neutralised by rubbing moist baking soda (sodium hydrogen carbonate) or calamine solution, which contains zinc carbonate.
Sodium hydrogen carbonate neutralizes the acid present in a bee's sting.
Explanation:
When we get stung by a bee, it releases a formic acid which irritates the affected area.And in order to relieve the irritation, we neutralize the formic acid by using sodium hydrogen carbonate which is basic in nature.Sodium hydrogen carbonate is basic in nature reacts with formic acid present in the sting to relieve the pain or irritation on the skin.The reaction between sodium hydrogen carbonate and formic acid occurs to give sodium formate, water, and carbon dioxide as a product\(NaHCO_3+HCOOH\rightarrow HCOONa+H_2O+CO_2\)
Learn more about the acid-base reaction here:
brainly.com/question/3069273?referrer=searchResults
brainly.com/question/15255706?referrer=searchResults
Which element rarely reacts because it has 8 electrons in its outer shell?
A. Bromine
B. Xenon
C. Arsenic
D. Potassium
Answers
Answer:
B. Xenon
Explanation:
A.P.E.X
According to the electronic configuration,the element which rarely reacts is xenon as it has 8 electrons in it's valence shell.
What is electronic configuration?Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/14283892
#SPJ5