a) Two ways to create a positive ion:
Answers
Positive ions are formed by atoms or molecules suffering an inelastic collision with an energetic electron in which an electron is lost from the atom or molecule (electron impact ionization). The degree of ionization of the plasma depends strongly on the electron density and energy distribution in the gas.
Related Questions
I didn't really understand the explanation, can it be broken down
Answers
Answer:
40.06
Explanation:
(23 * 39) + (48 * 40) + (29 * 41)
100
= 40.06
formula:
(% of isotope A * mass of A) + (% of isotope B * mass of B) ......
100
Answer:
yes it can be broken down
Which of the following is most easily reduced?
Click for a reduction potential chart
OA. Fe²+
OB. Zn+
OC. Agt
OD. Mg2+
SUBMIT
Answers
The reduction potential chart for metals, we can see that the most easily reduced metal in the list is Ag^+. Option C
What is reduction?A redox reaction is one that involves an oxidation and a reduction taking place simultaneously. This is because, the processes of electron loss and electron gain has to occur at the same time. A specie looses the electron that is gained by the other specie.
Now we know that metals are arranged in order of increasing reducing ability and decreasing reactivity. This arrangement is called the activity series of elements.
Looking at the reduction potential chart for metals, we can see that the most easily reduced metal in the list is Ag^+. Option C
Learn more about reduction potential:https://brainly.com/question/23881200
#SPJ1
Words are on the right please help!

Answers
Answer:
1.Frequency
2.Amplitude
3.Wavelength
4.Medium
5.Back and Forth
6.Up and Down
Explanation:
CORRECT ME IF I'm wrong
How do you convert kilograms to pounds?
Answers
Answer: (1 Kilogram = 2.20462 pounds) . There are 2.2046226218 lb in 1 kilogram. To convert kilograms to pounds, multiply your figure by 2.205 for an approximate result. 1 kilogram is also equal to 2 lb and 3.27396195 oz. Working out a rough estimate in your head for converting to pounds and ounces may be tricky - remember that there are 16 ounces in a pound.
The equation below shows the decomposition of lead nitrate. How many grams of lead (II) oxide are also produced when 20.5 g NO2 is formed?
Answers
Answer:
ligma
Explanation:
3 natural compounds of chlorine
Answers
How many total hydrogen atoms are there in the compound CH3(CH2)2CH3
Answers
Answer:
10 atoms of hydrogen
Explanation:
The atom of an element refers to the smallest indivisible substance of that element that retains its chemical properties. The atoms of an element combine to form a molecule, which then combines with atoms of other elements to form a compound.
In this case, CH3(CH2)2CH3 is a given organic compound. The number of hydrogen atoms can be determined thus:
H represents one hydrogen atom
- We have H3 - 3 atoms
- We have (H2)2 - 4 atoms
- We have H3 - 3 atoms
Altogether, this gives a total of 10 hydrogen atoms in the CH3(CH2)2CH3 compound.
3. The chemical formula of a mineral can be considered a statement about the chemical components and their proportions in a mineral's structure. One of the basic tenets is that the mineral must be electrically neutral. For each of the minerals listed below, write down the mineral formulae and list the valence (oxidation) state of cations and anions that make up that mineral.
2 | Page
EASC 219: Mineralogy Fall 2022
a. uvarovite
b. azurite
c. cuprite
d. gypsum
e. galena
Answers
The valence states provided are general representations and may vary depending on specific conditions and coordination environments.
a. Uvarovite: The mineral formula for uvarovite is Ca3Cr2(SiO4)3. In this formula, the valence state of calcium (Ca) is +2, the valence state of chromium (Cr) is +3, and the valence state of silicon (Si) is +4. Oxygen (O) is usually assigned a valence state of -2.
b. Azurite: The mineral formula for azurite is Cu3(CO3)2(OH)2. In this formula, the valence state of copper (Cu) is +2, carbonate (CO3) has a valence state of -2, and hydroxide (OH) has a valence state of -1.
c. Cuprite: The mineral formula for cuprite is Cu2O. In this formula, the valence state of copper (Cu) is +1, and oxygen (O) is usually assigned a valence state of -2.
d. Gypsum: The mineral formula for gypsum is CaSO4·2H2O. In this formula, the valence state of calcium (Ca) is +2, sulfur (S) has a valence state of +6, and oxygen (O) is usually assigned a valence state of -2. The water molecules (H2O) do not have a net charge.
e. Galena: The mineral formula for galena is PbS. In this formula, the valence state of lead (Pb) is +2, and sulfur (S) has a valence state of -2.
It's important to note that the valence states provided are general representations and may vary depending on specific conditions and coordination environments.
Learn more about valence from below link
https://brainly.com/question/371590
#SPJ11
Fluorine gas a 300 K occupies a volume of 500 mL. To what temperature should it be lowered to bring the volume to 300 mL?
Answers
V1/T1 = V2/T2
V1 = 500 mL
T1 = 300 K
V2 = 300 mL
Solve the equation for T2 —> T2 = V2T1/V1
T2 = (300 mL)(300 K) / (500 mL) = 180 K
The temperature should be lowered to 300K to bring the volume to 300mL.
What is temperature?Temperature is the degree of hotness or coldness of a place or a body. The SI unit of temperature is Kelvin.
What is volume?Volume is the amount of space occupied by matter. The SI unit of volume is cubic metre.
Using Charles law,
V1/V2 = T1/T2
Where, V1 and T1 are the initial volume and temperature
Similarly, V₂ and T₂ are the final volume and temperature.
Given:
V1 = 500mL
T1 = 300K
V2 = 300mL
T2 = ?
V1T2/V1 = V2T1/V1
T2 = V2T1/V1
= 300mL x 300K/500mL
T2 = 180K
Hence, the temperature should be lowered to 300K to bring the volume to 300mL.
To learn more about temperature and volume here
https://brainly.com/question/12050285
#SPJ2
What is the mass in grams of 3.45 moles of N?
Answers
mass of 1 mole of N is 14g
so mass of 3.45 mole is
3.45 × 14 = 48.3 g
What are three ways fluid flow is important in the food industry?
Answers
40. 0% carbon, 6. 7% hydrogen, and 53. 3% oxygen with a molecular mass of 60. 0 g/mol. What is the molecular formula of the unknown compound?
Answers
The molecular formula of the unknown compound is C2H2O2.
To determine the molecular formula of the unknown compound, we need to calculate the empirical formula first and then find the multiple of its subscripts to obtain the molecular formula.
Given:
Percentage of carbon = 40.0%
Percentage of hydrogen = 6.7%
Percentage of oxygen = 53.3%
Molecular mass = 60.0 g/mol
Step 1: Convert the percentages to grams.
Assuming we have 100 grams of the compound:
Mass of carbon = 40.0 g
Mass of hydrogen = 6.7 g
Mass of oxygen = 53.3 g
Step 2: Convert the masses to moles using the molar masses of the elements.
Molar mass of carbon = 12.01 g/mol
Molar mass of hydrogen = 1.008 g/mol
Molar mass of oxygen = 16.00 g/mol
Number of moles of carbon = Mass of carbon / Molar mass of carbon
= 40.0 g / 12.01 g/mol
= 3.332 mol
Number of moles of hydrogen = Mass of hydrogen / Molar mass of hydrogen
= 6.7 g / 1.008 g/mol
= 6.648 mol
Number of moles of oxygen = Mass of oxygen / Molar mass of oxygen
= 53.3 g / 16.00 g/mol
= 3.331 mol
Step 3: Determine the empirical formula by dividing the moles by the smallest value.
Dividing the moles of carbon, hydrogen, and oxygen by 3.331 gives approximately 1 for each element.
So, the empirical formula of the compound is CHO.
Step 4: Determine the multiple of the subscripts to obtain the molecular formula.
To find the multiple, we divide the molecular mass by the empirical formula mass.
Molecular mass = 60.0 g/mol
Empirical formula mass = (12.01 g/mol) + (1.008 g/mol) + (16.00 g/mol) = 29.018 g/mol
Multiple = Molecular mass / Empirical formula mass
= 60.0 g/mol / 29.018 g/mol
= 2.07
Rounding to the nearest whole number, we get 2.
Therefore, the molecular formula of the unknown compound is C2H2O2.
learn more about molecular here
https://brainly.com/question/30640129
#SPJ11
4 A 100g sample of water at 25°C is heated over a Bunsen burner until it nearly reaches boiling, at
99°C. How much heat (in joules) was applied to the beaker?
Answers
31,146.4 Joules of heat were applied to the beaker.
The amount of heat (q) required to heat a substance is given by:
q = m × c × ΔT
where:
m = mass of the substance
c = specific heat capacity of substance
ΔT = change in temperature
For water, the specific heat capacity (c) is 4.184 J/g°C.
The mass of water (m) is 100g.
The change in temperature (ΔT) is (99°C - 25°C) = 74°C.
Therefore, the amount of heat (q) required to heat the water is:
q = 100g × 4.184 J/g°C × 74°C
q = 31,146.4 J
Therefore, approximately 31,146.4 Joules of heat were applied to the beaker.
To know more about heat here
https://brainly.com/question/17039550
#SPJ4
What is the name of the compound with the chemical formula Br2F8?
Answers
The name of the compound with the chemical formula Br2F8 is dibromo octafluorine.
How chemical compounds are named?The chemical formula of a compound shows the type and proportion of each element that makes up the chemical compound.
According to this question, a compound with chemical formula Br2F8 is given. The compound consists of the following:
2 atoms of Bromine8 atoms of fluorineTherefore, the name of the compound with the chemical formula Br2F8 is dibromo octafluorine.
Learn more about nomenclature at: https://brainly.com/question/9837065?
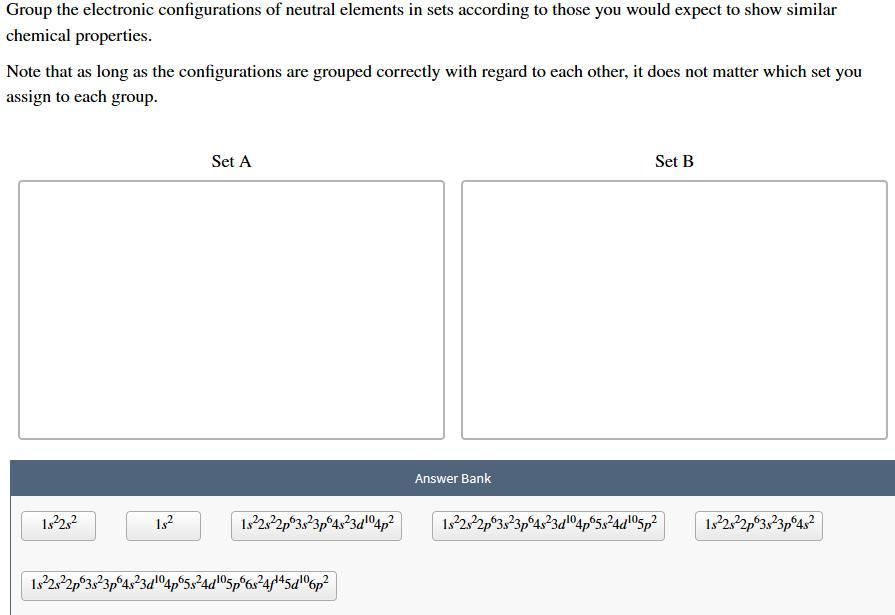
Group the electronic configurations of neutral elements in sets according to those you would expect to show similar chemical properties. Note that as long as the configurations are grouped correctly with regard to each other, it does not matter which set you assign to each group. Set A Set B Answer Bank 1:22:2 182 1922s22p03823p64323204p2 1322322p03823p 4323d"°4p65324d05p2 1922,22p3:23p6432 1.322s22p 3,23p64323 5p6s2445dº6p2
Answers
Set A elements behave similarly due to valence electrons in s and p orbitals, while Set B elements behave differently due to valence electrons in both s and d orbitals.
Set A:
1s²2s²
1s²
1s²2s²2p⁶3s²3p⁶4s²
Set B:
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p²
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p²
1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p²
The elements in Set A have valence electrons only in the s and p orbitals of their outermost energy level. This makes them similar in chemical behavior, as they tend to form covalent bonds by sharing electrons.
The elements in Set B have valence electrons in both the s and d orbitals of their outermost energy level, which gives them unique chemical properties, including the ability to form coordination complexes and exhibit variable oxidation states. Therefore, they are different in chemical behavior compared to the elements in Set A.
Learn more about neutral elements here: brainly.com/question/30433564
#SPJ4
Complete question is in the image attached below
Draw the structure (s) - 1-bromo-1-chloropropane show wedges and dashes. Draw highest Newman projection looking down th C1-C2 bond
Answers
1-Bromo-1-chloropropane has a bromine atom bonded to the first carbon (C1), a chlorine atom bonded to the second carbon (C2), and the remaining carbons connected in a chain. The highest Newman projection looking down the C1-C2 bond shows the C1 atom in the front, the C2 atom at the back, and the other atoms (Br, C3, and Cl) attached to the C1 atom.
Here's the structure of 1-bromo-1-chloropropane, showing wedges and dashes:
Br
|
C
/
C
/
C - Cl
To draw the highest Newman projection looking down the C1-C2 bond, we need to imagine looking along that bond with the C1 atom in front and the C2 atom at the back. The attached atoms (Br, C1, C3, and Cl) will be represented as circles.
Here's the highest Newman projection:
Br
|
C3
/
C1
/
C2
/
Cl
The C1 atom is represented by the intersection of the horizontal and vertical lines, while the C2 atom is shown as the circle at the end of the vertical line. The other atoms (Br, C3, and Cl) are attached to the C1 atom, and their positions are represented by their corresponding circles.
To learn more about Newman projection,
https://brainly.com/question/30167216
#SPJ4
What is the density of a glass fragment that has a mass of 1.5g and a volume of 0.75mL?
Answers
2gcm³ is the density of a glass fragment that has a mass of 1.5g and a volume of 0.75mL.
What is density ?The term density is define as the ratio of mass and volume. The formula for density is d = M/V, where d is density, M is mass, and V is volume. Density is commonly expressed in units of grams per cubic centimeter.
Density = mass / volume
Given:
Density = ?
Mass = 1.5 gram
Volume = 0.75 ml
By substituting this values in give equation we get,
Density = 1.5 / 0.75
= 2 gcm³
Thus, 2 gcm³ is the density of a glass fragment that has a mass of 1.5g and a volume of 0.75mL.
To learn more about the density, follow the link;
https://brainly.com/question/29775886
#SPJ1
which of the following statements about the kinetic-molecular theory of gases is false? 1. the average kinetic energy of a gas molecule is independent of the temperature. 2. collisions between molecules are elastic.
Answers
Kinetic molecular theory states that there is no attractive and repulsive force between the gas molecules. So option (3) is false.
According to the kinetic molecular theory the gases are composed of a large number of particles that behave like hard, spherical objects in a state of constant which is in random motion. This theory states that the energy that an object has because of its motion. The Kinetic Molecular Theory can be explained as the forces between molecules and the energy that they possess. This is explained as a theoretical model which describes the molecular composition of the gas in terms of a large number of submicroscopic particles that includes atoms and molecules. This states that the gas pressure arises due to particles colliding with each other and the walls of the container.
To learn more about Kinetic molecular theory
https://brainly.com/question/134712
#SPJ4
The complete question is,
Which of the following statements about the kinetic-molecular theory of gases is false?
1. the average kinetic energy of a gas molecule is independent of the temperature.
2. collisions between molecules are elastic.
3. Attractive and repulsive forces are present between gas molecule.
Curved arrows are provided for the transformation. Identify the products of the transformation. Be sure to draw nonbonding electrons and charges where appropriate.
Answers
Lewis Acid and Base :
Boron has only 3 electrons in its valence shell. But since the octet of boron is not complete, it still has the tendency to attract electrons. So, boron is lewis acid, while oxygen behaves as lewis base, as it has extra electrons on it, despite having its octet full.
What is Lewis Acid and Base ?A Lewis acid is a chemical species with an empty orbital that can accept an electron pair from a Lewis base to form a Lewis adduct. A Lewis base is defined as any species with a filled orbital containing an electron pair that is not involved in bonding but can form a dative bond with a Lewis acid to form a Lewis adduct.
Trimethylborane (Me3B) is a Lewis acid because it can accept a lone pair. A Lewis adduct is formed when the Lewis acid and base share an electron pair provided by the Lewis base, resulting in a dative bond.
Oxygen sends its lone pair of electrons to boron, thereby forming a coordinate bond. Initially, oxygen was holding 4 electrons all by itself. But now, it holds only 2 electrons by itself, while the electrons shared with boron are now held by both oxygen and boron, and so more stability has been gained.
A lone pair from NH3 will form a dative bond with the empty orbital of Me3B in the context of a specific chemical reaction between NH3 and Me3B to form the adduct NH3•BMe3. The terminology refers to Gilbert N. Lewis' contributions.
To learn more about Lewis Acid refer :
https://brainly.com/question/13263469
#SPJ4
TIME REMAINING
01:29:12
Which technology has helped improve scientists' ability to gather scientific data about the movement of sea turtles?
O satellite tracking
O thermometers
O probeware
microscopes
Answers
the first one satellite tracking
Answer:
I believe the answer is A. Or the 1st dot.
Explanation:
How does heat transfer by radiation different from the heat transfer by conduction or convection
Answers
The major difference between heat transfer by conduction and convection and heat transfer by radiation is that heat transfer by conduction and radiation requires a material medium, while heat transfer by radiation is done by electromagnetic waves and doesn't require a medium.
Heat or thermal energy is the energy generated when atoms or molecules move in a variety of directions (translational, rotational and vibrational). The three methods of heat transfer are convection, conduction and radiation. Conduction only occurs between solid objects in contact, convection occurs within a fluid, and radiation occurs through electromagnetic waves.
Unlike conduction and convection, radiation doesn't require a material medium before heat is transferred, as electromagnetic radiations don't require a material medium to propagate. Heat is transferred by radiation through infrared rays. Other differences include:
Conduction and convection are slow, while radiation is fast, as infrared rays travel at the speed of light.Radiation can be reflected using mirrors, while conduction and convection cannot.Learn more about radiation here:
https://brainly.com/question/10219972
#SPJ4
WHAT DO YOU UNDERSTAND BY OLFACTORY INDICATORS?
Answers
Answer:
An Olfactory Indicator is a chemical that changes it's scent. This depends on whether it is mixed with an acid or base solution. When mixed, it's called "olfactory titration."
Explanation:
Hope I helped :)
Free Brainly !! What is the cation and its' charge for the following compound?
CaCl2
Answers
Calcium ions have a charge of +2, while chloride ions have a charge of -1. The molecule for calcium chloride has one calcium ion (+2) and two chloride ions (-1), which means that the overall charge for the molecule is 0, or neutral.
calcium cation
Formula and structure: The chemical formula of calcium chloride is CaCl2, and its molar mass is 110.983 g/mol. It is an ionic compound consisting of the calcium cation (Ca2+) and two chlorine anions (Cl-). The bivalent calcium metal forms an ionic bond with two chlorine atoms, as shown below.
Answer:
calcium cation
Formula and structure: The chemical formula of calcium chloride is CaCl2, and its molar mass is 110.983 g/mol. It is an ionic compound consisting of the calcium cation (Ca2+) and two chlorine anions (Cl-).
Explanation:
C6H12O6 + 6 O2(g) 6 CO2(g) + 6 H2O(g)
If 7.5 g of glucose react with 5.5 L of oxygen, which one is the limiting reagent?
Answers
C₆H₁₂O₆ + 6O₂ ---> 6CO₂ + 6H₂O, If 7.5 g of glucose react with 5.5 L of oxygen, the limiting reagent is glucose.
The balanced reaction is given as :
C₆H₁₂O₆ + 6O₂ ---> 6CO₂ + 6H₂O
mass of glucose = 7.5 g
molar mass of glucose = 180 g/mol
number of moles =mass / molar mass
= 7.5 / 180
= 0.041 mol
volume of oxygen = 5.5 L
density of oxygen = 1.42 g/ml
mass = density × volume
mass = 1.42 × 5.5
mass = 7.81
moles = mass / molar mass
= 7.81 / 32
= 0.244 mol
mass of reactant from each product:
now, glucose , mass of CO₂ = 0.041 × 6 × 44 = 10.84 g
oxygen, mass of CO₂ = 0.244 × 6 × 44 = 64.41 g
Since glucose gives the smaller amount of product glucose is a limiting reagent.
Thus, C₆H₁₂O₆ + 6O₂ ---> 6CO₂ + 6H₂O, If 7.5 g of glucose react with 5.5 L of oxygen, the limiting reagent is glucose.
To learn more about limiting reagent here
https://brainly.com/question/26905271
#SPJ1
cs-137 is produced as a waste product in nuclear fission reactors. what fraction remains undecayed after 241.84 years?
Answers
After 241.84 years, only about 3.2% of the original amount of Cs-137 remains undecayed. Proper management and disposal of nuclear waste products are crucial to prevent harm to the environment and human health.
Cesium-137 (Cs-137) is a radioactive isotope that is produced as a fission product in nuclear reactors. It has a half-life of about 30 years, which means that after each 30-year period, half of the Cs-137 will decay into a stable element. Therefore, to determine the fraction of Cs-137 that remains undecayed after 241.84 years, we can use the following formula:
Fraction remaining = \(\left(\frac{1}{2}\right)^{\frac{t}{h}}\)
where t is the time elapsed and h is the half-life of Cs-137.
In this case, t is 241.84 years and h is 30 years, so we can substitute these values into the formula and calculate the fraction remaining:
Fraction remaining = \(\left(\frac{1}{2}\right)^{\frac{241.84}{30}}\)
Fraction remaining ≈ 0.032
Therefore, after 241.84 years, only about 3.2% of the original amount of Cs-137 remains undecayed. The remaining 96.8% has decayed into stable isotopes. This highlights the importance of properly managing and disposing of nuclear waste products to avoid potential harm to the environment and human health.
To learn more about nuclear
https://brainly.com/question/18187269
#SPJ4
Which of the following is considered the first receiver for the nicotine in a smoking person?
1.left atrium
2.right atrium
3.left ventricle
4.right ventricle
Answers
Answer:
i think right artrium but not full aure
Exactly 10.0 L of air -25°C is heated to 100.0°C. What is the new volume if the pressure is kept constant?
Answers
Answer: V2= 15.0403226 Liters
Explanation:
Use V1/T1=V2/T2
Make sure you change the degrees Celsius to Kelvin. (Kelvin = degrees Celsius +273)
10.0L / 248 K = V2/ 373 K
Cross multiply V1 and T2 and divide by T1
(10.0 L)( 373K)/ 248 K = V2
V2= 15.0403226 Liters (Kelvin cancels out)

Inside Earth, it is very hot and this source of heat called
Answers
Answer:
Geothermal energy
Explanation:
Hope that helps !!!
Based on the information shown, which of the following explains why the pressure in tank X is greater than that in tank Y ?
Tank X tank y
Ar(g) N2(g)
p=150atm p=130atm
v=70L v=70L
T=21 c T=21c
Answers
The pressure in tank X is greater than that in tank Y because the number of moles or mass of argon gas in Tank X is greater than the number of moles or mass of nitrogen gas in Tank Y.
What is the pressure of a gas?The pressure of a gas is the force that molecules of the gas exert per unit area of the walls of their container due the collisions of the gas molecules with themselves and with the walls of their contain.
The pressure of a gas is related to the volume (V), temperature (T) and number of moles (n) of the gas by the formula:
P = nRT/Vwhere;
R is molar gas constantn = mass/Molar mass.Since volume, temperature and R is constant for the two gases, the pressure difference will be determined by the number of moles of each gas.
Number of moles of a gas is proportional to the mass of the gas present.
Therefore, the best explanation as to why the pressure in tank X is greater than that in tank Y is that the number of moles or mass of argon gas in Tank X is greater than the number of moles or mass of nitrogen gas in Tank Y.
Learn more about gas pressure at: https://brainly.com/question/25736513
theoretically, if you were to use one serving of the food you chose to start a fire under a pot of water, what volume of water could you bring to a boil at 100 °c if all of the fire's energy was absorbed by the water?
Answers
The volume of water that could be brought to a boil using one serving of the chosen food depends on the energy content of the food and the heat energy required to boil the water.
To determine the volume of water that could be brought to a boil at 100 °C using one serving of a chosen food as the fuel source, we need to consider the energy content of the food and the energy required to heat the water.
First, we need to identify the chosen food and its energy content. Different foods have different energy densities, which can be measured in units such as calories or joules per serving. Let's assume the chosen food has an energy content of X calories per serving.
The energy required to raise the temperature of water can be calculated using the specific heat capacity of water. The specific heat capacity of water is approximately 4.18 joules per gram per degree Celsius (J/g°C).
To calculate the volume of water, we need to convert the energy content of the food to joules and divide it by the energy required to raise the temperature of water. Since the energy content of food is typically given in calories, we need to convert calories to joules. One calorie is equivalent to 4.18 joules.
Let's assume one serving of the chosen food provides Y calories. The energy content in joules would be Y * 4.18 joules.Now, we can calculate the volume of water using the equation:
Volume of water = Energy content of food (in joules) / (Energy required to heat water * Change in temperature)
Let's assume we want to bring the water to a boil, which requires raising the temperature from room temperature (assumed to be 25 °C) to 100 °C. Therefore, the change in temperature would be 75 °C.
Volume of water = (Y * 4.18) / (4.18 * 75)
Simplifying the equation, we find that the volume of water that could be brought to a boil using one serving of the chosen food as the fuel source is:
Volume of water = Y / 75
For more such question on actual volume visit:
https://brainly.com/question/30986210
#SPJ8