A tank of air is at 125 psi, and currently sitting outside at 35 c, or 308 kelvin. the tank has a maximum pressure rating of 165psi if you were to start heating up the air tank at what temperature would the tank be at risk of rupturing
Answers
If you were to start heating up the air tank, it would be at risk of rupturing when the temperature reaches approximately 404.16 Kelvin or 131.01 degrees Celsius.
To determine the temperature at which the air tank would be at risk of rupturing, we can use the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature.
Given:
Initial pressure (P1) = 125 psi
Initial temperature (T1) = 35 °C = 308 K
Maximum pressure rating (P2) = 165 psi
We can set up the following equation using the ideal gas law:
(P1/T1) = (P2/T2)
Solving for T2 (temperature at risk of rupturing):
T2 = (P2 * T1) / P1
Substituting the values:
T2 = (165 psi * 308 K) / 125 psi
≈ 404.16 K
Therefore, if you were to start heating up the air tank, it would be at risk of rupturing when the temperature reaches approximately 404.16 Kelvin or 131.01 degrees Celsius.
learn more about Celsius here
https://brainly.com/question/14767047
#SPJ11
Related Questions
give the orbital configuration of the following elements using the s, p, d, f type representation. (answer format is: 1se2 = 1s 2 ) helium, nitrogen, silicon helium nitrogen silicon
Answers
These orbital configurations represent the arrangement of electrons within the different energy levels and subshells of the respective elements.
The orbital configurations of the given elements are as follows:
Helium: 1s² - Helium has two electrons that occupy the 1s orbital.
Nitrogen: 1s² 2s² 2p³ - Nitrogen has two electrons in the 1s orbital, two electrons in the 2s orbital, and three electrons in the 2p orbital (specifically, 2p³ indicates three electrons in the 2p subshell).
Silicon: 1s² 2s² 2p⁶ 3s² 3p² - Silicon has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, two electrons in the 3s orbital, and two electrons in the 3p orbital (specifically, 3p² indicates two electrons in the 3p subshell).
To know more about the orbital configurations refer here :
https://brainly.com/question/13840107#
#SPJ11
Question 1 (1 point) What is white light? The type of light that is found in x-rays The type of light given off by the sun and light bulbs known as visible light. The type of light that is used in microwave ovens They type of light that makes up radio wave Submit
Answers
apparently colorless light, for example ordinary daylight. It contains all the wavelengths of the visible spectrum at equal intensity.
Hey there!!
Radio waves, on the other hand, have the lowest energies, longest wavelengths, and lowest frequencies of any type of EM radiation. In order from highest to lowest energy, the sections of the EM spectrum are named: gamma rays, X-rays, ultraviolet radiation, visible light, infrared radiation, and radio waves.
Hope It Helped!~
JustinBiberLovers~
Which joint in the human body is similar to the chicken wing joint?
Answers
The joint in the human body which is similar to the chicken wing joint is elbow joint. There is some similarities in the arm joint movement of chicken and humans.
What is joint?Joints are the connecting parts for bones. Thus two bones are connected by joints. The skeletal system is a web of different living tissues that gives the human body its structure and protects organs.
Additionally, it is where blood cells are created. Due to their similar evolutionary history as vertebrates, chicken wings are homologous to the upper limb of humans; that is, they share many of the same features.
In order to connect the shape of muscles, bones, and joints to their function, we can analyze a chicken wing. The structure, tissues and some movements of elbow joint of humans is similar to that of chicken wing joint.
To find more about joints, refer the link below:
https://brainly.com/question/15093622
#SPJ1
L. Complete this nuclear equation for the alpha decay of Uus-294 by writing a
notation for the missing product:

Answers
The nuclear equation for the alpha decay of Uus-294 is
\(^{294}{117} Uus\) →\(^{4}{2}He + ^{290}_{115}Mc\)
To complete the nuclear equation for the alpha decay of Uus-294, we need to determine the missing product.
During alpha decay, the emission of an alpha particle, composed of two protons and two neutrons, leads to the formation of a new nucleus.
The balanced nuclear equation for the alpha decay of Uus-294 can be represented as follows:
\(^{294}{117} Uus\) → \(^{4}{2}He +\)_____(missing product)
In this equation, the atomic number of the missing product must be two less than the atomic number of Uus-294 (117 - 2 = 115), and the mass number of the missing product must be four less than the mass number of Uus-294 (294 - 4 = 290).
Based on this information, the missing product can be identified as:
\(^{290}_{115}Mc\)
Mc stands for Moscovium, which has an atomic number of 115. By subtracting two from the atomic number of Uus, we obtain the atomic number of Mc. The mass number of Mc-290 is obtained by subtracting four from the mass number of Uus-294.
Therefore, the nuclear equation for the alpha decay of Uus-294 is:
\(^{294}{117} Uus\)→ \(^{4}{2}He + ^{290}_{115}Mc\)
Know more about alpha decay here:
https://brainly.com/question/25714324
#SPJ8
The complete question is :
Complete this nuclear equation for the alpha decay of Uus-294 by writing a
notation for the missing product:
\(^{204} {117} Uns\)→\(^{4} _{2} He +\)_____
complete the measure correctly with a single note. ch4 q38 group of answer choices dotted eighth note eighth note sixteenth note quarter note
Answers
The measure can be correctly completed with a quarter note. This note gets one beat in the 4/4 time signature. The is that it is important to understand time signatures and note values when completing a measure. A measure is a segment of music that is separated by bar lines.
It is also called a bar. In Western music, there are a few different time signatures. The most common is 4/4 time. The top number indicates how many beats are in each measure, while the bottom number indicates what type of note gets one beat. A quarter note gets one beat in 4/4 time.
It is also sometimes called a crotchet. The other answer choices, dotted eighth note, eighth note, and sixteenth note, have different lengths and do not fit within one beat in 4/4 time. Therefore, a quarter note is the correct choice to complete the measure correctly with a single note. The measure can be completed correctly with a quarter note. In 4/4 time signature, a quarter note gets one beat.
To know more about values Visit;
https://brainly.com/question/10053178
#SPJ11
Identify the compound proposition involving the propositional variables p, q, and r that is true when p and q are true and r is false, but is false otherwise.
Answers
These statements contradict each other, hence C is true if and only if one of the three statements is true
Think about the following: C=(p∧q∧¬r)∨(p∧¬q∧r)∨(¬p∧q∧r)
The outer disjunction () is used in this compound statement C, and it states that it is true if and only if one of the three propositions (p∧q∧¬r)∨(p∧¬q∧r)∨(¬p∧q∧r) is true.
First, it is not conceivable for two or three of these statements to be true at the same time. For instance, if (p∧q∧¬r) and (p∧¬q∧r)are both true, then (r, from the first conjuncture) and (r, from the second conjuncture), respectively, are true, which is a contradiction. The same logic can be used to rule out any other possibility.
To learn more about Contradiction :
https://brainly.com/question/17439687
#SPJ4
When filling a buret for a titration, first adjust the buret in the clamp so that ______________ then, choose... to add the titrant into the buret. the titrant should be filled ___________
Answers
When filling a buret for a titration, first adjust the buret in the clamp so that it is vertical. Then, choose an appropriate funnel to add the titrant into the buret. The titrant should be filled to just above the zero mark on the buret, and the buret tip should be briefly opened to remove any bubbles.
After that, the buret can be adjusted to the desired volume and the titration can proceed. It is important to take note of the initial buret reading before starting the titration to ensure accurate measurement of the titrant volume.
When filling a buret for a titration, it is important to first adjust the buret in the clamp so that it is vertical and its tip is below eye level to ensure accurate volume measurements. Next, choose an appropriate method to add the titrant into the buret, such as using a funnel or a pipette.
It is important to avoid splashing or spilling the titrant to ensure accurate and precise measurements. The titrant should be filled above the zero mark on the buret and then slowly drained until the bottom of the meniscus is aligned with the zero mark.
This process is called "buret priming" and it helps to remove any air bubbles from the buret tip that can affect the accuracy of the measurements. Once the buret is primed and filled with the titrant, it is ready to be used for the titration.
For more question on titration click on
https://brainly.com/question/16839748
#SPJ11
1 volume of gas X react with exactly 5 volumes of oxygen, carbon dioxide and water are produced. what is the gas X?
Answers
The gas X is C₃H₈, which is propane.
Organic substances known as hydrocarbons only produce CO2 and water when they burn. So, by the hit and trial method of x and y in the general formula of the reaction mentioned, we get propane.
A three-carbon alkane, propane has the chemical formula C3H8. At room temperature and pressure, it is a gas, but it can be compressed into a liquid for transportation. It is a by-product of the processing of natural gas and the refining of petroleum and is frequently used as fuel.
To learn more about propane,
https://brainly.com/question/14519324
An isotope of an element has a different number of _____.
neutrons
protons
electrons
Answers
I believe that the answer is
A) Neutrons
I hope this helps you ^-^
Answer:
neutrons
Explanation:
A metallic element, M, reacts vigorously with water to form a solution of MOH. If M is in Period 4, what is the valence-shell configuration of the atom? (Express your answer as a series of valence orbitals. For example, the valence-shell configuration of Li would be entered as 2s1.)
Answers
The valence-shell configuration of the metallic element M in Period 4 is 4s2 4p6.
What is the valence-shell configuration of the metallic element M in Period 4?
The valence-shell configuration refers to the arrangement of electrons in the outermost shell, or valence shell, of an atom. In Period 4, the valence shell of the metallic element M would be the fourth shell, denoted as the 4s and 4p orbitals.
The electron configuration of an element is determined by the position of the element in the periodic table. Since M is in Period 4, we know that it has four electron shells. The valence electrons are those located in the outermost shell, which determine the element's chemical properties and reactivity.
In this case, the valence-shell configuration of M is given as 4s2 4p6, indicating that there are two electrons in the 4s orbital and six electrons in the 4p orbitals. The total number of valence electrons can be calculated as the sum of the electrons in the valence orbitals, which in this case is 8.
Learn more about valence-shell
brainly.com/question/20861869
#SPJ11
The picture below shows a sample from a tree.
What kind of tree is this?
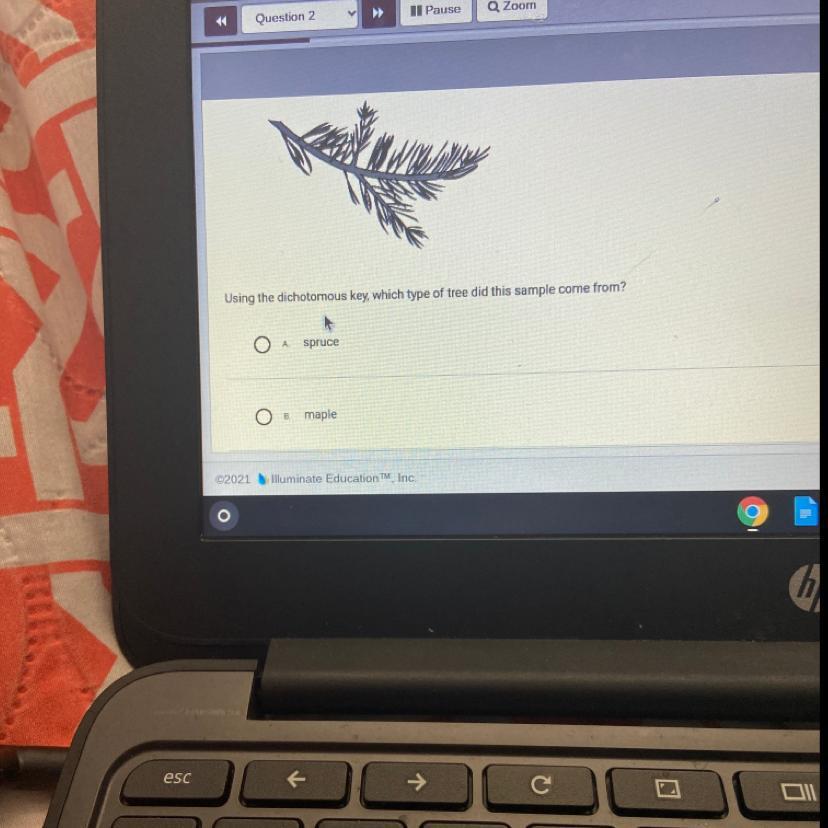
Answers
Answer:
I believe it is Spruce, because of the shape of the leaves
Answer:
Explanation:
Spruce
Two scientists are debating how to classify a new animal species that they have discovered. They observe that the animal is capable of producing sperm.
What statement would they most likely make about the new species?
Answers
Answer:
This animal produces offspring that are genetically different from itself.
Explanation:
Answer:
answer is A
This animal produces offspring that are genetically different from itself.
Explanation:
hope this helps!
A balloon contains 14.0 L of air at a pressure of 760 torr. What will the volume of the air be when the balloon is taken to a depth of 10ft in a swimming pool, where the pressure is 981 torr? The temperature of the air in the balloon doesn't change. a) 8.8 L
b) 17.7 L
c) 15.4 L
d) 10.8 L
Answers
the final volume of air in the balloon when the balloon is taken to a depth of 10ft in a swimming pool, where the pressure is 981 torr is 10.8 L. Answer: d) 10.8 L.
We are given the initial volume of air in the balloon, Vi = 14.0 L. The initial pressure, Pi = 760 torr. The final pressure, Pf = 981 torr. The depth of the swimming pool, h = 10 ft. The temperature of the air, T is constant, which means that the gas in the balloon is an ideal gas.
We can use Boyle's law and the pressure difference to find the final volume of air.Boyle's law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. That is,V_1/P_1 = V_2/P_2where V1 and P1 are the initial volume and pressure, and V2 and P2 are the final volume and pressure.
Rearranging this equation, we getV_2 = V_1 × P_1/P_2= 14.0 L × (760 torr)/(981 torr)= 10.8 L
Therefore, the final volume of air in the balloon is 10.8 L. Answer: d) 10.8 L.
To learn more about volume visit;
https://brainly.com/question/28058531
#SPJ11
what trend in iconic radius do you see as you down a group/family on the periodic table
Answers
Answer:
ionic radii of elements increases down the group
Explanation:
Although at ionic state elements acquire noble gas configuration, there is an extra energy level down the group.
This leads to a decrease in attraction between electrons and the nucleus down the group.
For advanced level
Down the group an extra energy level creates screening of the penultimate shell decreasing the effective nuclear charge.
This leads to increase in the radius
How does the radius of calcium compare to the radius of potassium?
Answers
Answer:
Calcium has a smaller radius in comparison to Potassium.
Explanation:
When we move down a group, the atomic radius increases, and when we move from left to right in a period, the atomic radius decreases. Since both Potassium and Calcium are in the 4th period, we have to look left to right. Calcium is to the right of Potassium making the radius smaller.
the reaction rate constant is determined to be 0.012 m-1 s-1. if after 27 minutes the amount of a left is 0.048 m. what was the initial concentration of a?
Answers
The initial concentration of 'A' was approximately 1.371 M.
To determine the initial concentration of 'A' given the reaction rate constant, time, and the remaining amount of 'A', you can use the integrated rate law equation for a first-order reaction:
[A]t = [A]₀ * e^(-kt)
where:
[A]t = concentration of 'A' at time t (0.048 M)
[A]₀ = initial concentration of 'A'
k = reaction rate constant (0.012 M⁻¹ s⁻¹)
t = time in seconds (27 minutes = 27 * 60 = 1620 seconds)
Now, let's solve for [A]₀:
0.048 M = [A]₀ * e^(-0.012 M⁻¹ s⁻¹ * 1620 s)
To find [A]₀, divide both sides by e^(-0.012 * 1620):
[A]₀ = 0.048 M / e^(-0.012 * 1620)
Now, calculate the value:
[A]₀ ≈ 0.048 M / e^(-19.44)
[A]₀ ≈ 0.048 M / 0.000035
[A]₀ ≈ 1.371 M
So, the initial concentration of A was approximately 1.371 M.
Learn more about rate constant here:
brainly.com/question/31327496
#SPJ11
derive a formula for the time t that it will take for the perfume molecules to diffuse a distance l into the room. you can assume that the mass m and collision cross-section σ of the molecules of perfume are roughly the same as those of air molecules; that is, you can assume that m is the same for the perfume, o2, and n2, and likewise for σ. hint: the answer will depend on l, m, σ, the pressure p, the temperature t.
Answers
The formula for the time (t) it will take for perfume molecules to diffuse a distance (l) into the room can be derived as follows: t = (l^2) / (6D), where D is the diffusion coefficient.
Diffusion is the process by which molecules spread out from an area of high concentration to an area of low concentration. In this case, we are considering the diffusion of perfume molecules into the room. To derive a formula for the time it takes for diffusion to occur, we need to consider the factors that affect the rate of diffusion.
The time it takes for molecules to diffuse a distance (l) can be related to the diffusion coefficient (D), which is a measure of how quickly molecules move and spread out. The formula for the time (t) can be derived using the equation t = (l^2) / (6D), where (l^2) represents the squared distance traveled and 6D represents the diffusion coefficient.
The diffusion coefficient depends on various factors, including the mass (m) and collision cross-section (σ) of the perfume molecules, as well as the pressure (p) and temperature (t) of the environment. By assuming that the mass and collision cross-section of the perfume molecules are similar to air molecules, we can consider them to be constant in the formula.
It's important to note that this derived formula is a simplification and assumes ideal conditions. Real-world diffusion processes may involve additional factors and complexities. However, the derived formula provides a starting point for understanding the relationship between diffusion time, distance, and the diffusion coefficient.
Learn more about Molecules
brainly.com/question/32298217
brainly.com/question/30465503
#SPJ11
I need a description of the rocks.
Table D. Absolute Age of Rock Layers

Answers
Basalt is 65.5 million years ago to the present. Limestone with fossil was about 500 million years ago. Sandstone with trilobite is between 525 and 505 million years old.
What is Absolute Age of rocks?A quantifiable measurement of how old something is or how long ago it occurred, expressed typically in terms of years, is called an absolute age in geology.
Radiometric techniques are used the most often in geology to determine absolute ages.
Basalt was formed 65.5 million years ago. Fossilized limestone dates back to roughly 500 million years. Trilobites are found in sandstone that is between 525 and 505 million years old.
In the Carboniferous epoch, about 340 million years ago, the earliest amniotes diverged from their amphibian predecessors. Soon after the first amniotes emerged, they split into two major lines.
Thus, these are the probable ages of the given rocks.
For more details regarding absolute age, visit:
https://brainly.com/question/29770777
#SPJ1
what is empirical formula for CLO2
Answers
Answer:
chlorine dioxide is empirical formula for CLO2.
Metalliods have _______ proporties of metals and non metals.
a
both
b
neither
c
different
Answers
Answer:
A both
Explanation:
hope this helped
Q. Explain why is sulfuric acid a corrosive?
.
.
.
.
.
.
Can we clean a dumb humans brain with windex?if yes then tell me how?
Answers
Answer: yes
Explanation: cut open their head and spray their head with windex. THAT SIMPLE
A cinder block sits on a platform 20 m high. If it has a mass of 8 kg, find its energy.
Answers
There is a cinder block on a 20-meter-high platform. If it has a mass of 8 kg The energy is 1568 joules.
What do you mean by mass?a numerical representation of the fundamental characteristic of all matter, inertia. In essence, it's the resistance of a mass of matter to changing its direction or speed in reaction to the application of a force. The more mass a body possesses, the smaller the change that is brought about by an applied force.
How would you measure mass?The formula F = m a, where F is force, m is mass, and an is acceleration, can be used to calculate mass. Determine the force, which is equivalent to weight, the acceleration, which is equivalent to gravity, and the mass.
Calculation:mass= 8 kg
height= 20 m
acceleration= 9.8
The energy can be calculated as follows
= mgh
= 8×9.8×20
= 1568
The energy is 1568 joules.
To know more about Mass visit:
https://brainly.com/question/19694949
#SPJ9
. Balance the equations and identify the type of reaction. (48 points total: 6 points per question=2
points per reaction type and 4 points for the coefficients)
a. ___Na3N → ___Na + ___N2 Reaction Type:
b. ___H3PO4 + ___KOH → ___K3PO4 + ___H2O Reaction Type:
c. ___N2 + ___H2 → ___NH3 Reaction Type:
d. ___H2O2 → ___ O2 + ___ H2O Reaction Type:
e. ___Zn+ ___HCl → ___ZnCl2 + ___ H2 Reaction Type:
f. ___C2H6 + ___O2 → ___ CO2 + ___H2O Reaction Type:
g. ___CuCl2 + ___ H2S → ___CuS + ___HCl Reaction Type:
h. ___N2 + ___ O2 → ___ N2O5 Reaction Type:
Answers
The balanced equation of the reaction and the reaction types are given below as follows:
a. 2 Na₃N → 6 Na + N₂ Reaction Type: decomposition reaction
b. H₃PO₄ + 3 KOH → K₃PO₄ + 3 H₂O Reaction Type: Neutralization reaction
c. N₂ + 3 H₂ → 2 NH₃ Reaction Type: Synthesis
d. 2 H₂O₂ → O₂ + 2 H₂O Reaction Type: Decomposition
e. Zn+ 2 HCl → ZnCl₂ + H₂ Reaction Type: single displacement
f. 2 C₂H₆ + 7 O₂ → 4 CO₂ + 6 H₂O Reaction Type: Combustion
g. CuCl₂ + H₂S → CuS + 2 HCl Reaction Type: Double displacement
h. 2 N₂ + 5 O2 → 2 N₂O₅ Reaction Type: Synthesis
What are chemical reactions?Chemical reactions are reactions that result in the formation of new substances as a result of chemical changes that occur in the substances undergoing the reaction.
In a chemical reaction, the substances that undergo chemical changes are called reactants while the new substances that are formed are called products.
A balanced equation of a chemical reaction is one in which the moles of atoms of reactants are equal to the moles of atoms of the products involved in the reaction.
Learn more about chemical reactions at: https://brainly.com/question/16416932
#SPJ1
PLEASE TYPED 150 WORD MIN Describe a radioactive isotope that exists in nature. Provide details on what the product is the half-life, what the daughter products are and the type of decay process that occurs. Discuss whether the daughter product or products are stable or unstable. Be sure to cite your source.
Answers
One example of a naturally occurring radioactive isotope is uranium-238 (U-238).
Uranium-238 (U-238) is a radioactive isotope that exists in nature. Its half-life is approximately 4.5 billion years. U-238 undergoes a decay process called alpha decay, where it emits an alpha particle consisting of two protons and two neutrons. The daughter product of U-238 decay is thorium-234 (Th-234), which is also radioactive with a half-life of about 24 days. Th-234 further decays into protactinium-234 (Pa-234) through beta decay.
The decay chain continues until it reaches a stable isotope, lead-206 (Pb-206). The daughter products of U-238 decay are unstable and undergo further radioactive decay until a stable isotope is reached.
Source: "Uranium-238." Los Alamos National Laboratory, https://periodic.lanl.gov/92.shtml
For more questions like Radioactive isotope click the link below:
https://brainly.com/question/15266093
#SPJ11
Question 3 (1 point)
If a 8.50 grams of a hydrate of sodium carbonate, Na2CO3, is heated so that the mass then becomes 7.26 grams, what is the correct formula of
the hydrate? Na2CO3 _H20
Blank 1:
Question 4 (1 point)
Which is the formula for a hydrate of calcium sulfate, CaSO4, that had a mass of 60.98 grams before heating and 48.23 grams after heating?
CaSO4
_H20
Blank 1:
Answers
Answer:
1. Formulae of hydrate is Na₂CO₃.10H₂O
2. Formulae of hydrate is CaSO₄.2H₂O
Explanation:
Q1. Mass of hydrated salt = 8.50 g mass
mass of anhydrous salt = 7.26 grams
mass of water lost = 1.24
Formula of hydrated salt = Na₂CO₃.xH₂O
Formula of anhydrous salt = Na₂CO₃
Molar mass of anhydrous salt is obtained as below where Na = 23, C = 12, O = 16, H = 1
Molar mass of Na₂CO₃ = (23 *2 + 12 + 16 * 3) = 106 g
molar mass of water = (1 *2 + 16) = 18 g
Mole ratio of Na₂CO₃ to H₂O = 7.26/106 : 1.24/18
Mole ratio of Na₂CO₃ to H₂O = 0.0068 : 0.068
Mole ratio of Na₂CO₃ to H₂O = 1 :10
Therefore, formulae of hydrate is Na₂CO₃.10H₂O
Q2. Mass of hydrated salt = 60.98 g mass
mass of anhydrous salt = 48.23 grams
mass of water lost = 12.75
Formula of hydrated salt = CaSO₄.xH₂O
Formula of anhydrous salt = CaSO₄
Molar mass of anhydrous salt is obtained as below where Ca = 40, S = 32, O = 16, H = 1
Molar mass of CaSO₄= (40 + 32 + 16 * 4) = 136 g
molar mass of water = (1 *2 + 16) = 18 g
Mole ratio of CaSO₄ to H₂O = 48.23/136 : 12.75/18
Mole ratio of CaSO₄ to H₂O = 0.35 : 0.70
Mole ratio of CaSO₄ to H₂O = 1 : 2
Therefore, formulae of hydrate is CaSO₄.2H₂O
Explanation:
Question 3:
Mass of anhydrous compound = 7.26g
Mass of water = 8.50 - 7.26 = 1.24 g
Percentage of water of crystallization in the compound is = (1.24 / 8.50) * 100 = 14.59%
The mass ratio of Na2CO3 : H2O = 7.26 : 1.24
Using mole = mass / molar mass;
The mole ratio of Na2CO3 : H2O = 7.26 /105.99 : 1.24 / 18 = 0.069 : 0.069 or 1 : 1
The formular is; Na2CO3.H2O
Question 4:
Mass of anhydrous compound = 48.23 g
Mass of water = 60.98 - 48.23 = 12.75 g
Percentage of water of crystallization in the compound is = (12.75 / 60.98) * 100 = 20.91%
The mass ratio of CaSO4 : H2O = 48.23 : 12.75
Using mole = mass / molar mass;
The mole ratio of CaSO4 : H2O = 48.23 / 136.14 : 12.75 / 18 = 0.3543 : 0.708 which is 1 : 2
The formular is; CaSO4.2H2O
What nuclear reaction could hydrogen undergo, fission or fusion?*
Answers
Answer:
Hydrogen (H) “burning” initiates the fusion energy source of stars and leads to the formation of helium (He).
FUSION Reaction
Explanation:
mark me brainliest please.
an unknown compound contains only c , h , and o . combustion of 5.90 g of this compound produced 13.4 g co2 and 5.49 g h2o . what is the empirical formula of the unknown compound? insert subscripts as needed.
Answers
The unidentified compound's empirical formula is C9.45H5.13O1 (rounded to the nearest whole number). The simplest whole number ratio of the atoms in a compound is the empirical formula.
We must ascertain the relative proportions of each element in the combination in order to get the empirical formula. the volume of CO2 generated: 13.4 g of CO2 are created per mass of C. mass of H = (mass of H2O created) (2 mol H2O / 1 mol H2) (1 g/mol) = 5.49 g 2 / 18.015 g/mol = 0.610 g. the compound's overall bulk 5.90 g, 13.4 g, 0.610 g, and 1.89 g are the mass of O, total mass, mass of C, and mass of H, respectively. figuring out the empirical formula Create moles out of the masses: 13.4 g divided by 12.011 g/mol yields 1.117 mol of carbon. 0.606 mol is equal to 0.610 g/1.008 g/mol, or moles of H. Omoles are calculated as 1.89 g / 15.999 g/mol, or 0.118 mol. the most basic ratio of entire numbers: moles of C, moles of H, and moles of O are equal to 1.117, 0.606, and 0.118 mol/moles, respectively. The abbreviation is: C9.45H5.13O1. Hence, C9.45H5.13O1 is the empirical formula for the unidentified molecule.
Learn more about empirical formula here:
https://brainly.com/question/29506551
#SPJ4
please answer all parts thank you
Complete the simple analysis of temperature (for which there are always observations of temperature that correspond to the contour values) in Figure 2 for the 75 and 70°F isotherms. The 80°F contour
Answers
Given Figure 2 below shows a set of contour lines for temperature, and the question wants you to complete a simple analysis of temperature. The analysis should be made for the 75 and 70°F isotherms. The 80°F contour is also to be analyzed.
Figure 2 From the image above, we can identify the following contour lines and their values:Contour line C1 is for a temperature of 60°F.Contour line C2 is for a temperature of 65°F.Contour line C3 is for a temperature of 70°F.Contour line C4 is for a temperature of 75°F.Contour line C5 is for a temperature of 80°F.Using the given information, we can then proceed to answer the questions as follows:Analysis for the 75°F isotherm Contour line C4 shows a temperature of 75°F. This means that any point lying on this contour line has a temperature value of 75°F. Therefore, we can conclude that the following regions have a temperature of 75°F:Region A: This region is enclosed by contour lines C3 and C4.
Thus, it has a temperature of 75°F.Region B: This region is enclosed by contour lines C4 and C5. Thus, it has a temperature of 75°F.Analysis for the 70°F isotherm Contour line C3 shows a temperature of 70°F. This means that any point lying on this contour line has a temperature value of 70°F. Therefore, we can conclude that the following regions have a temperature of 70°F:Region C: This region is enclosed by contour lines C2 and C3. Thus, it has a temperature of 70°F.Region D: This region is enclosed by contour lines C3 and C4. Thus, it has a temperature of 70°F.Analysis for the 80°F contourContour line C5 shows a temperature of 80°F. This means that any point lying on this contour line has a temperature value of 80°F. Therefore, we can conclude that the following regions have a temperature of 80°F:Region E: This region is enclosed by contour lines C4 and C5. Thus, it has a temperature of 80°F.
To know more about point lying visit:-
https://brainly.com/question/30174758
#SPJ11
name any group 2 element in chemistry periodic table
Answers
Explanation:
The Alkaline Earth Metals. Group 2A (or IIA) of the periodic table are the alkaline earth metals: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
to the best of your knowledge, classify each of the following as an element, compound, or mixture. explain how your everyday experiences influenced your response. a. silver coin b. air c. coffee d. soil
Answers
a. Silver coin - Element (Silver is a pure element and is not chemically combined with any other element in a silver coin)
b. Air - Mixture (Air is a mixture of gases, primarily nitrogen and oxygen, with trace amounts of other gases and particles)
c. Coffee - Mixture (Coffee is a mixture of various compounds such as water, caffeine, and organic compounds that give it its flavour and aroma)
d. Soil - Mixture (Soil is a mixture of various substances such as minerals, organic matter, water, and air)
My everyday experiences influenced my response because I have come across these examples in my daily life and have been taught about them in science classes. For example, I know that air is composed of different gases and particles, and that soil is made up of a mixture of substances, including minerals and organic matter. Similarly, I know that a silver coin is made of pure silver and that coffee is made of various compounds.
To learn more about Compounds click here:
https://brainly.com/question/14658388
#SPJ4